Tsg101 control of human immunodeficiency virus type 1 Gag trafficking and release
- PMID: 12915533
- PMCID: PMC187429
- DOI: 10.1128/jvi.77.17.9173-9182.2003
Tsg101 control of human immunodeficiency virus type 1 Gag trafficking and release
Abstract
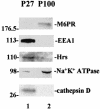
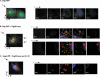
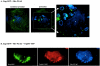
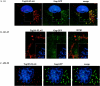
The structural precursor polyprotein of human immunodeficiency virus type 1, Pr55(gag), contains a proline-rich motif (PTAP) called the "late domain" in its C-terminal p6 region that directs release of mature virus-like particles (VLPs) from the plasma membranes of gag-transfected COS-1 cells. The motif binds Tsg101 (vacuolar protein-sorting protein 23, or Vps23), which functions in endocytic trafficking. Here, we show that accumulation of the wild-type (wt) Gag precursor in a fraction of COS-1 cytoplasm enriched in multivesicular bodies and small particulate components of the plasma membrane (P100) is p6 dependent. Cleavage intermediates and mature CA mainly partitioned with more rapidly sedimenting larger material enriched in components of lysosomes and early endosomes (P27), and this also was p6 dependent. Expression of truncated or full-length Tsg101 proteins interfered with VLP assembly and Gag accumulation in the P100 fraction. This correlated with reduced accumulation of Gag tagged with green fluorescent protein (Gag-GFP) at the plasma membrane and colocalization with the tagged Tsg101 in perinuclear early endosomes, as visualized by confocal microscopy. Fractionation analysis and confocal examination both indicated that the N-terminal region of Tsg101, which contains binding sites for PTAP and ubiquitin (Ub), was required for Gag trafficking to the plasma membrane. Expression of FLAG-tagged Tsg101 with a deletion in the Ub-binding pocket inhibited VLP release almost completely and to a significantly greater extent than expression of the wt tagged Tsg101 protein or Tsg101-FLAG containing a deletion in the PTAP-binding region. The results demonstrate that Gag associates with endosomal trafficking compartments and indicate that efficient release of virus particles from the plasma membrane requires both the PTAP- and Ub-binding functions of Tsg101 to recruit the cellular machinery required for budding.
Figures




References
-
- Alland, L., S. M. Peseckis, R. E. Atherton, L. Berthiaume, and M. D. Resh. 1994. Dual myristylation and palmitylation of Src family member p59fyn affects subcellular localization. J. Biol. Chem. 269:16701-16705. - PubMed
-
- Babst, M., D. J. Katzmann, E. J. Estepa-Sabal, T. Meerloo, and S. D. Emr. 2002. ESCRT-III: an endosome-associated heterooligomeric protein complex required for MVB sorting. Dev. Cell 3:271-282. - PubMed
-
- Babst, M., D. J. Katzmann, W. B. Synder, B. Wendland, and S. D. Emr. 2002. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell 3:282-289. - PubMed
-
- Babst, M., G. Odorizzi, E. J. Estepa, and S. D. Emr. 2000. Mammalian tumor susceptibility gene 101 (Tsg101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic 1:248-258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

