Association of the herpes simplex virus type 1 Us11 gene product with the cellular kinesin light-chain-related protein PAT1 results in the redistribution of both polypeptides
- PMID: 12915535
- PMCID: PMC187382
- DOI: 10.1128/jvi.77.17.9192-9203.2003
Association of the herpes simplex virus type 1 Us11 gene product with the cellular kinesin light-chain-related protein PAT1 results in the redistribution of both polypeptides
Abstract
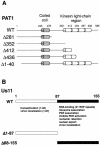
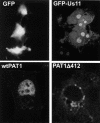
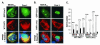
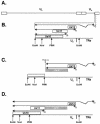
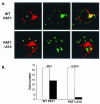
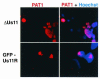
The herpes simplex virus type 1 (HSV-1) Us11 gene encodes a multifunctional double-stranded RNA (dsRNA)-binding protein that is expressed late in infection and packaged into the tegument layer of the virus particle. As a tegument component, Us11 associates with nascent capsids after its synthesis late in the infectious cycle and is delivered into newly infected cells at times prior to the expression of viral genes. Us11 is also an abundant late protein that regulates translation through its association with host components and contains overlapping nucleolar retention and nuclear export signals, allowing its accumulation in both nucleoli and the cytosol. Thus, at various times during the viral life cycle and in different intracellular compartments, Us11 has the potential to execute discrete tasks. The analysis of these functions, however, is complicated by the fact that Us11 is not essential for viral replication in cultured cells. To discover new host targets for the Us11 protein, we searched for cellular proteins that interact with Us11 and have identified PAT1 as a Us11-binding protein according to multiple, independent experimental criteria. PAT1 binds microtubules, participates in amyloid precursor protein trafficking, and has homology to the kinesin light chain (KLC) in its carboxyl terminus. The carboxyl-terminal dsRNA-binding domain of Us11, which also contains the nucleolar retention and nuclear export signals, binds PAT1, whereas 149 residues derived from the KLC homology region of PAT1 are important for binding to Us11. Both PAT1 and Us11 colocalize within a perinuclear area in transiently transfected and HSV-1-infected cells. The 149 amino acids derived from the KLC homology region are required for colocalization of the two polypeptides. Furthermore, although PAT1 normally accumulates in the nuclear compartment, Us11 expression results in the exclusion of PAT1 from the nucleus and its accumulation in the perinuclear space. Similarly, Us11 does not accumulate in the nucleoli of infected cells that overexpress PAT1. These results establish that Us11 and PAT1 can associate, resulting in an altered subcellular distribution of both polypeptides. The association between PAT1, a cellular trafficking protein with homology to KLC, and Us11, along with a recent report demonstrating an interaction between Us11 and the ubiquitous kinesin heavy chain (R. J. Diefenbach et al., J. Virol. 76:3282-3291, 2002), suggests that these associations may be important for the intracellular movement of viral components.
Figures



References
-
- Bowman, A. B., A. Kamal, B. W. Ritchings, A. V. Philp, M. McGrail, J. G. Gindhart, and L. S. Goldstein. 2000. Kinesin-dependent axonal transport is mediated by the sunday driver (SYD) protein. Cell 103:583-594. - PubMed
-
- Bresnahan, W. A., and T. Shenk. 2000. A subset of viral transcripts packaged within human cytomegalovirus particles. Science 288:2373-2376. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

