Production of human papillomavirus type 16 virus-like particles in transgenic plants
- PMID: 12915537
- PMCID: PMC187377
- DOI: 10.1128/jvi.77.17.9211-9220.2003
Production of human papillomavirus type 16 virus-like particles in transgenic plants
Abstract
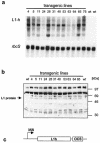
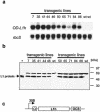
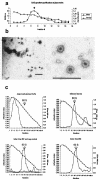
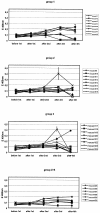
Cervical cancer is linked to infection with human papillomaviruses (HPV) and is the third most common cancer among women worldwide. There is a strong demand for the development of an HPV preventive vaccine. Transgenic plants expressing the HPV major capsid protein L1 could be a system to produce virus-like particles for prophylactic vaccination or could even be used as edible vaccines to induce an L1-specific prophylactic immune response. Here, we describe the generation of transgenic tobacco and potato plants carrying the HPV type 16 major structural gene L1 under the control of the cauliflower mosaic virus 35S promoter. All attempts to express either the original, unmodified L1 gene or an L1 gene with a codon usage optimized for expression in plants failed. Surprisingly, small amounts of the protein were detected using an L1 gene optimized for expression in human cells. However, Northern blot analysis revealed that most of the L1 transcripts were degraded. Introduction of the translational enhancer Omega derived from the tobacco mosaic virus strongly increased transcript stability and resulted in accumulation of L1 protein to approximately 0.5 to 0.2% of total soluble protein in transgenic tobacco and potato plants, respectively. The plant-derived L1 protein displayed conformation-specific epitopes and assembled into virus-like particles. Furthermore, we did not find any indications of protein modification of the L1 protein produced in plants. Plant-derived L1 was as immunogenic as L1 expressed in baculovirus-infected insect cells. Feeding of tubers from transgenic potatoes to mice induced an anti-L1 antibody response in 3 out of 24 mice, although this response was only transient in two of the mice. Our data, however, indicate that an anti-L1 response was primed in about half of the 24 animals.
Figures



Similar articles
-
Expression of HPV-11 L1 protein in transgenic Arabidopsis thaliana and Nicotiana tabacum.BMC Biotechnol. 2007 Sep 12;7:56. doi: 10.1186/1472-6750-7-56. BMC Biotechnol. 2007. PMID: 17850660 Free PMC article.
-
Enhancement of capsid gene expression: preparing the human papillomavirus type 16 major structural gene L1 for DNA vaccination purposes.J Virol. 2001 Oct;75(19):9201-9. doi: 10.1128/JVI.75.19.9201-9209.2001. J Virol. 2001. PMID: 11533183 Free PMC article.
-
Vaccine regimen for prevention of sexually transmitted infections with human papillomavirus type 16.Vaccine. 2001 May 14;19(25-26):3583-90. doi: 10.1016/s0264-410x(01)00070-6. Vaccine. 2001. PMID: 11348726
-
Papillomavirus-like particles for serology and vaccine development.Intervirology. 1996;39(1-2):54-61. doi: 10.1159/000150475. Intervirology. 1996. PMID: 8957670 Review.
-
Developments in L2-based human papillomavirus (HPV) vaccines.Virus Res. 2017 Mar 2;231:166-175. doi: 10.1016/j.virusres.2016.11.020. Epub 2016 Nov 23. Virus Res. 2017. PMID: 27889616 Free PMC article. Review.
Cited by
-
Scalable Production of HPV16 L1 Protein and VLPs from Tobacco Leaves.PLoS One. 2016 Aug 12;11(8):e0160995. doi: 10.1371/journal.pone.0160995. eCollection 2016. PLoS One. 2016. PMID: 27518899 Free PMC article.
-
Expression of codon optimized major capsid protein (L1) of human papillomavirus type 16 and 18 in Pichia pastoris; purification and characterization of the virus-like particles.Vaccine. 2011 Oct 6;29(43):7326-34. doi: 10.1016/j.vaccine.2011.07.071. Epub 2011 Jul 29. Vaccine. 2011. PMID: 21803095 Free PMC article.
-
Immunotherapy of HPV-associated cancer: DNA/plant-derived vaccines and new orthotopic mouse models.Cancer Immunol Immunother. 2015 Oct;64(10):1329-38. doi: 10.1007/s00262-015-1734-0. Epub 2015 Jul 3. Cancer Immunol Immunother. 2015. PMID: 26138695 Free PMC article. Review.
-
Chimeric Human Papillomavirus-16 Virus-like Particles Presenting P18I10 and T20 Peptides from HIV-1 Envelope Induce HPV16 and HIV-1-Specific Humoral and T Cell-Mediated Immunity in BALB/c Mice.Vaccines (Basel). 2022 Dec 21;11(1):15. doi: 10.3390/vaccines11010015. Vaccines (Basel). 2022. PMID: 36679860 Free PMC article.
-
HPV vaccine: an overview of immune response, clinical protection, and new approaches for the future.J Transl Med. 2010 Oct 27;8:105. doi: 10.1186/1479-5876-8-105. J Transl Med. 2010. PMID: 20979636 Free PMC article. Review.
References
-
- Arakawa, T., J. Yu, D. K. Chong, J. Hough, P. C. Engen, and W. H. Langridge. 1998. A plant-based cholera toxin B subunit-insulin fusion protein protects against the development of autoimmune diabetes. Nat. Biotechnol. 16:934-938. - PubMed
-
- Bosch, F. X., M. M. Manos, N. Munoz, M. Sherman, A. M. Jansen, J. Peto, M. H. Schiffman, V. Moreno, R. Kurman, K. V. Shah, et al. 1995. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J. Natl. Cancer Inst. 87:796-802. - PubMed
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
-
- Breitburd, F., R. Kirnbauer, N. L. Hubbert, B. Nonnenmacher, C. Trin-Dinh-Desmarquet, G. Orth, J. T. Schiller, and D. R. Lowy. 1995. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J. Virol. 69:3959-3963. - PMC - PubMed
-
- Chargelegue, D., P. Obregon, and P. M. Drake. 2001. Transgenic plants for vaccine production: expectations and limitations. Trends Plant Sci. 6:495-496. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

