Human cytomegalovirus US2 causes similar effects on both major histocompatibility complex class I and II proteins in epithelial and glial cells
- PMID: 12915544
- PMCID: PMC187418
- DOI: 10.1128/jvi.77.17.9287-9294.2003
Human cytomegalovirus US2 causes similar effects on both major histocompatibility complex class I and II proteins in epithelial and glial cells
Abstract
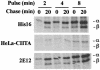
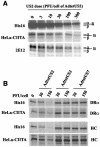
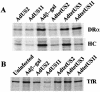
The human cytomegalovirus (HCMV) glycoprotein US2 specifically binds to major histocompatibility complex (MHC) class I heavy chain (HC) and class II proteins DRalpha and DMalpha, triggering their degradation by proteasomes. Effects of US2 on class II proteins were originally characterized in HCMV- or adenovirus vector-infected U373 astroglioma cells. Here, we have extended characterization of US2-mediated degradation of class II DRalpha to two other cell lines, including biologically relevant epithelial cells. Comparison of the effects of US2 in cells expressing both class I and II proteins demonstrated only a slight preference for class I HC. Moreover, US2 caused degradation of DRalpha and DMalpha when these proteins were expressed by transfection without DRbeta, invariant chain (Ii), or DMbeta. Therefore, US2 binds to alpha chains of DR and DM and triggers endoplasmic reticulum degradation without formation of class II DR alphabeta/Ii or DM alphabeta complexes. Similar levels of degradation of class II alpha were observed in cells expressing vastly different amounts of class II, suggesting that cellular factors, other than class II, were limiting. We concluded that US2 has broad effects in a variety of cells that express both class I and II proteins and is relevant to HCMV infection in vivo.
Figures



References
-
- Ahn, K., A. Gruhler, B. Galocha, T. R. Jones, E. J. Wiertz, H. L. Ploegh, P. A. Peterson, Y. Yang, and K. Fruh. 1997. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity 6:613-621. - PubMed
-
- Ben-Arieh, S. V., B. Zimerman, N. I. Smorodinsky, M. Yaacubovicz, C. Schechter, I. Bacik, J. Gibbs, J. R. Bennink, J. W. Yewdell, J. E. Coligan, H. Firat, F. Lemonnier, and R. Ehrlich. 2001. Human cytomegalovirus protein US2 interferes with the expression of human HFE, a nonclassical class I major histocompatibility complex molecule that regulates iron homeostasis. J. Virol. 75:10557-10562. - PMC - PubMed
-
- Bitmansour, A. D., S. L. Waldrop, C. J. Pitcher, E. Khatamzas, F. Kern, V. C. Maino, and L. J. Picker. 2001. Clonotypic structure of the human CD4+ memory T cell response to cytomegalovirus. J. Immunol. 167:1151-1163. - PubMed
-
- Borza, C. M., and L. M. Hutt-Fletcher. 2002. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat. Med. 8:594-599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

