Novel recombinant parapoxvirus vectors induce protective humoral and cellular immunity against lethal herpesvirus challenge infection in mice
- PMID: 12915547
- PMCID: PMC187421
- DOI: 10.1128/jvi.77.17.9312-9323.2003
Novel recombinant parapoxvirus vectors induce protective humoral and cellular immunity against lethal herpesvirus challenge infection in mice
Abstract
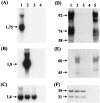
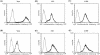
Orf virus (ORFV; Parapoxvirus ovis) was used to develop a novel vector system for the generation of effective and safe live vaccines. Based on the attenuated ORFV strain D1701-V, recombinants were produced that express the glycoproteins gC (D1701-VrVgC) or gD (D1701-VrVgD) of the alphaherpesvirus of swine, pseudorabies virus (PRV). Expression of gC and gD was also demonstrated on the surface of recombinant virus-infected murine cells that do not produce infectious ORFV. Single or combined immunization with the ORFV recombinants protected different mouse strains of a host species nonpermissive for ORFV against a fulminant, lethal PRV challenge infection equal to immunization with PRV live vaccine. Most notably, even a single immunization with D1701-VrVgC was protective, whereas two applications of D1701-VrVgD were required for immune protection. The higher protective capacity of D1701-VrVgC correlated with the induction of a strong specific humoral immune response. This suggestion was supported by transfer experiments using sera from recombinant-immunized mice, which resulted in partial gC but not gD antibody-mediated protection of the naïve recipients. Remarkably, immunization of different immune-deficient mice demonstrated that the application of the PRV gC-expressing recombinant controlled the challenge infection in the absence of either CD4(+) or CD8(+) T cells, B cells, or an intact perforin pathway. In contrast, D1701-VrVgD-immunized mice lacking CD4(+) T cells exhibited reduced protection, whereas animals lacking CD8(+) T cells, B cells, or perforin resisted the challenge infection. The present study demonstrates the potential of these new vector vaccines to efficiently prime both protective humoral and cell-mediated immune mechanisms in a host species nonpermissive for the vector virus.
Figures


References
-
- Ben Porat, T., J. M. DeMarchi, B. Lomniczi, and A. S. Kaplan. 1986. Role of glycoproteins of pseudorabies virus in eliciting neutralizing antibodies. Virology 154:325-334. - PubMed
-
- Bianchi, A. T., H. W. Moonen-Leusen, F. J. van Milligen, H. F. Savelkoul, R. J. Zwart, and T. G. Kimman. 1998. A mouse model to study immunity against pseudorabies virus infection: significance of CD4+ and CD8+ cells in protective immunity. Vaccine 16:1550-1558. - PubMed
-
- Brenner, G. J., N. Cohen, and J. A. Moynihan. 1994. Similar immune response to nonlethal infection with herpes simplex virus-1 in sensitive (BALB/c) and resistant (C57BL/6) strains of mice. Cell. Immunol. 157:510-524. - PubMed
-
- Büttner, M., and H. J. Rziha. 2002. Parapoxviruses: from the lesion to the viral genome. J. Vet. Med. B Infect. Dis. Vet. Public Health 49:7-16. - PubMed
-
- Cooney, E. L., A. C. Collier, P. D. Greenberg, R. W. Coombs, J. Zarling, D. E. Arditti, M. C. Hoffman, S. L. Hu, and L. Corey. 1991. Safety of and immunological response to a recombinant vaccinia virus vaccine expressing HIV envelope glycoprotein. Lancet 337:567-572. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

