Latent herpes simplex virus infection of sensory neurons alters neuronal gene expression
- PMID: 12915567
- PMCID: PMC187408
- DOI: 10.1128/jvi.77.17.9533-9541.2003
Latent herpes simplex virus infection of sensory neurons alters neuronal gene expression
Abstract
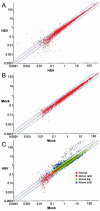
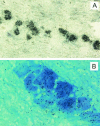
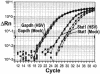
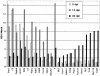
The persistence of herpes simplex virus (HSV) and the diseases that it causes in the human population can be attributed to the maintenance of a latent infection within neurons in sensory ganglia. Little is known about the effects of latent infection on the host neuron. We have addressed the question of whether latent HSV infection affects neuronal gene expression by using microarray transcript profiling of host gene expression in ganglia from latently infected versus mock-infected mouse trigeminal ganglia. (33)P-labeled cDNA probes from pooled ganglia harvested at 30 days postinfection or post-mock infection were hybridized to nylon arrays printed with 2,556 mouse genes. Signal intensities were acquired by phosphorimager. Mean intensities (n = 4 replicates in each of three independent experiments) of signals from mock-infected versus latently infected ganglia were compared by using a variant of Student's t test. We identified significant changes in the expression of mouse neuronal genes, including several with roles in gene expression, such as the Clk2 gene, and neurotransmission, such as genes encoding potassium voltage-gated channels and a muscarinic acetylcholine receptor. We confirmed the neuronal localization of some of these transcripts by using in situ hybridization. To validate the microarray results, we performed real-time reverse transcriptase PCR analyses for a selection of the genes. These studies demonstrate that latent HSV infection can alter neuronal gene expression and might provide a new mechanism for how persistent viral infection can cause chronic disease.
Figures
References
-
- Adamec, E., P. S. Mohan, A. M. Cataldo, J. P. Vonsattel, and R. A. Nixon. 2000. Up-regulation of the lysosomal system in experimental models of neuronal injury: implications for Alzheimer's disease. Neuroscience 100:663-675. - PubMed
-
- Baldi, P., and A. D. Long. 2001. A Bayesian framework for the analysis of microarray expression data: regularized t-test and statistical inferences of gene changes. Bioinformatics 17:509-519. - PubMed
-
- Bartke, A., V. Chandrashekar, D. Turyn, R. W. Steger, L. Debeljuk, T. A. Winters, J. A. Mattison, N. A. Danilovich, W. Croson, D. R. Wernsing, and J. J. Kopchick. 1999. Effects of growth hormone overexpression and growth hormone resistance on neuroendocrine and reproductive functions in transgenic and knock-out mice. Proc. Soc. Exp. Biol. Med. 222:113-123. - PubMed
-
- Benovic, J. L., J. J. Onorato, J. L. Arriza, W. C. Stone, M. Lohse, N. A. Jenkins, D. J. Gilbert, N. G. Copeland, M. G. Caron, and R. J. Lefkowitz. 1991. Cloning, expression, and chromosomal localization of beta-adrenergic receptor kinase 2. A new member of the receptor kinase family. J. Biol. Chem. 266:14939-14946. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

