Specific association of glycoprotein B with lipid rafts during herpes simplex virus entry
- PMID: 12915568
- PMCID: PMC187402
- DOI: 10.1128/jvi.77.17.9542-9552.2003
Specific association of glycoprotein B with lipid rafts during herpes simplex virus entry
Abstract
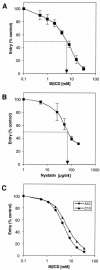
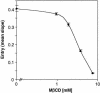
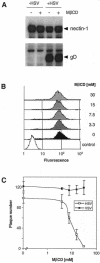
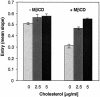
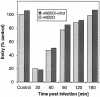
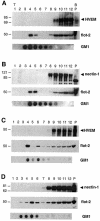
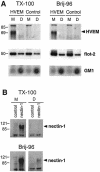
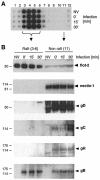
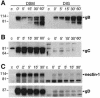
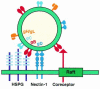
Herpes simplex virus (HSV) entry requires the interaction of glycoprotein D (gD) with a cellular receptor such as herpesvirus entry mediator (HVEM or HveA) or nectin-1 (HveC). However, the fusion mechanism is still not understood. Since cholesterol-enriched cell membrane lipid rafts are involved in the entry of other enveloped viruses such as human immunodeficiency virus and Ebola virus, we tested whether HSV entry proceeds similarly. Vero cells and cells expressing either HVEM or nectin-1 were treated with cholesterol-sequestering drugs such as methyl-beta-cyclodextrin or nystatin and then exposed to virus. In all cases, virus entry was inhibited in a dose-dependent manner, and the inhibitory effect was fully reversible by replenishment of cholesterol. To examine the association of HVEM and nectin-1 with lipid rafts, we analyzed whether they partitioned into nonionic detergent-insoluble glycolipid-enriched membranes (DIG). There was no constitutive association of either receptor with DIG. Binding of soluble gD or virus to cells did not result in association of nectin-1 with the raft-containing fractions. However, during infection, a fraction of gB but not gC, gD, or gH associated with DIG. Similarly, when cells were incubated with truncated soluble glycoproteins, soluble gB but not gC was found associated with DIG. Together, these data favor a model in which HSV uses gB to rapidly mobilize lipid rafts that may serve as a platform for entry and cell signaling. It also suggests that gB may interact with a cellular molecule associated with lipid rafts.
Figures
References
-
- Amici, C., G. Belardo, A. Rossi, and M. G. Santoro. 2001. Activation of IκB kinase by herpes simplex virus type 1. A novel target for anti-herpetic therapy. J. Biol. Chem. 276:28759-28766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

