Antiviral activity of limitin against encephalomyocarditis virus, herpes simplex virus, and mouse hepatitis virus: diverse requirements by limitin and alpha interferon for interferon regulatory factor 1
- PMID: 12915574
- PMCID: PMC187381
- DOI: 10.1128/jvi.77.17.9622-9631.2003
Antiviral activity of limitin against encephalomyocarditis virus, herpes simplex virus, and mouse hepatitis virus: diverse requirements by limitin and alpha interferon for interferon regulatory factor 1
Abstract
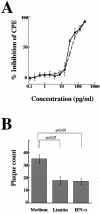
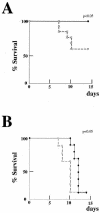
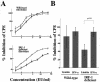
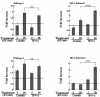
Limitin has sequence homology with alpha interferon (IFN-alpha) and IFN-beta and utilizes the IFN-alpha/beta receptor. However, it has no influence on the proliferation of normal myeloid and erythroid progenitors. In this study, we show that limitin has antiviral activity in vitro as well as in vivo. Limitin inhibited not only cytopathic effects in encephalomyocarditis virus- or herpes simplex virus (HSV) type 1-infected L929 cells, but also plaque formation in mouse hepatitis virus (MHV) type 2-infected DBT cells. In addition, administration of limitin to mice suppressed MHV-induced hepatitis and HSV-induced death. The antiviral activity may be mediated in part by 2',5'-oligoadenylate synthetase, RNA-dependent protein kinase, and Mx protein, which inhibit viral replication or degrade viral components, because limitin induced their mRNA expression and enzyme activity. While limitin has antiviral activity as strong as that of IFN-alpha in vitro (the concentration that provided 50% inhibition of cytopathic effect is approximately 30 pg/ml), IFN regulatory factor 1 (IRF-1) dependencies for induction of an antiviral state were different for limitin and IFN-alpha. In IRF-1-deficient fibroblasts, a higher concentration of limitin than of IFN-alpha was required for the induction of antiviral activity and the transcription of proteins from IFN-stimulated response element. The unique signals and the fewer properties of myelosuppression suggest that a human homolog of limitin may be used as a new antiviral drug.
Figures
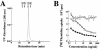


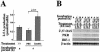
Similar articles
-
A new interferon, limitin, displays equivalent immunomodulatory and antitumor activities without myelosuppressive properties as compared with interferon-alpha.Exp Hematol. 2004 Sep;32(9):797-805. doi: 10.1016/j.exphem.2004.06.008. Exp Hematol. 2004. PMID: 15345280
-
The immediate-early protein, ICP0, is essential for the resistance of herpes simplex virus to interferon-alpha/beta.Virology. 2002 Feb 15;293(2):295-304. doi: 10.1006/viro.2001.1280. Virology. 2002. PMID: 11886249
-
Effect of deficiency of the double-stranded RNA-dependent protein kinase, PKR, on antiviral resistance in the presence or absence of ribonuclease L: HSV-1 replication is particularly sensitive to deficiency of the major IFN-mediated enzymes.J Interferon Cytokine Res. 2000 Jul;20(7):653-9. doi: 10.1089/107999000414835. J Interferon Cytokine Res. 2000. PMID: 10926208
-
Limitin: an interferon-like cytokine without myeloerythroid suppressive properties.J Mol Med (Berl). 2001 May;79(4):168-74. doi: 10.1007/s001090100206. J Mol Med (Berl). 2001. PMID: 11409707 Review.
-
IFN-zeta/ limitin: a member of type I IFN with mild lympho-myelosuppression.J Cell Mol Med. 2005 Apr-Jun;9(2):244-54. doi: 10.1111/j.1582-4934.2005.tb00353.x. J Cell Mol Med. 2005. PMID: 15963247 Free PMC article. Review.
Cited by
-
Tumor Restrictions to Oncolytic Virus.Biomedicines. 2014 Apr 17;2(2):163-194. doi: 10.3390/biomedicines2020163. Biomedicines. 2014. PMID: 28548066 Free PMC article. Review.
-
The antiviral activity of arbidol hydrochloride against herpes simplex virus type II (HSV-2) in a mouse model of vaginitis.Int Immunopharmacol. 2019 Mar;68:58-67. doi: 10.1016/j.intimp.2018.09.043. Epub 2019 Jan 3. Int Immunopharmacol. 2019. PMID: 30612085 Free PMC article.
-
IFN-ε is constitutively expressed by cells of the reproductive tract and is inefficiently secreted by fibroblasts and cell lines.PLoS One. 2013 Aug 9;8(8):e71320. doi: 10.1371/journal.pone.0071320. eCollection 2013. PLoS One. 2013. PMID: 23951133 Free PMC article.
-
Interferon-zeta/limitin: novel type I interferon that displays a narrow range of biological activity.Int J Hematol. 2004 Nov;80(4):325-31. doi: 10.1532/ijh97.04087. Int J Hematol. 2004. PMID: 15615256 Review.
-
Inhibition of Beta interferon induction by severe acute respiratory syndrome coronavirus suggests a two-step model for activation of interferon regulatory factor 3.J Virol. 2005 Feb;79(4):2079-86. doi: 10.1128/JVI.79.4.2079-2086.2005. J Virol. 2005. PMID: 15681410 Free PMC article.
References
-
- Arnheiter, H., M. Frese, R. Kambadur, E. Meier, and O. Haller. 1996. Mx transgenic mice—animal models of health. Curr. Top. Microbiol. Immunol. 206:119-147. - PubMed
-
- Cebrian, M., E. Yague, M. O. de Landazuri, M. Rodiguez-Moya, M. Fresno, N. Pezzi, S. Llamazares, and F. Sanchez-Madrid. 1987. Different functional sites on rIFN-alpha 2 and their relation to the cellular receptor binding site. J. Immunol. 138:484-490. - PubMed
-
- Clemens, M. J., and B. R. Williams. 1978. Inhibition of cell-free protein synthesis by pppA2′p5′A2′p5′A: a novel oligonucleotide synthesized by interferon-treated L cell extracts. Cell 13:565-572. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

