Rapamycin causes down-regulation of CCR5 and accumulation of anti-HIV beta-chemokines: an approach to suppress R5 strains of HIV-1
- PMID: 12915736
- PMCID: PMC193575
- DOI: 10.1073/pnas.1834278100
Rapamycin causes down-regulation of CCR5 and accumulation of anti-HIV beta-chemokines: an approach to suppress R5 strains of HIV-1
Abstract
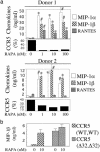
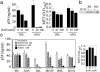
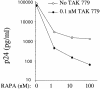
Propagation of R5 strains of HIV-1 on CD4 lymphocytes and macrophages requires expression of the CCR5 coreceptor on the cell surface. Individuals lacking CCR5 (CCR5 Delta 32 homozygous genotype) are phenotypically normal and resistant to infection with HIV-1. CCR5 expression on lymphocytes depends on signaling through the IL-2 receptor. By FACS analysis we demonstrate that rapamycin (RAPA), a drug that disrupts IL-2 receptor signaling, reduces CCR5 surface expression on T cells at concentrations as low as 1 nM. In addition, lower concentrations of RAPA (0.01 nM) were sufficient to reduce CCR5 surface expression on maturing monocytes. PCR analysis on peripheral blood mononuclear cells (PBMCs) showed that RAPA interfered with CCR5 expression at the transcriptional level. Reduced expression of CCR5 on PBMCs cultured in the presence of RAPA was associated with increased extracellular levels of macrophage inflammatory protein (MIP)-1 alpha and MIP-1 beta. In infectivity assays, RAPA suppressed the replication of R5 strains of HIV-1 both in PBMC and macrophage cultures. In total PBMC cultures, RAPA-mediated inhibition of CCR5-using strains of HIV-1 occurred at 0.01 nM, a concentration of drug that is approximately 103 times lower than therapeutic through levels of drug in renal transplant recipients. In addition, RAPA enhanced the antiviral activity of the CCR5 antagonist TAK-779. These results suggest that low concentrations of RAPA may have a role in both the treatment and prevention of HIV-1 infection.
Figures


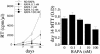
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

