Effect of Aplidin in acute lymphoblastic leukaemia cells
- PMID: 12915891
- PMCID: PMC2376915
- DOI: 10.1038/sj.bjc.6601130
Effect of Aplidin in acute lymphoblastic leukaemia cells
Abstract
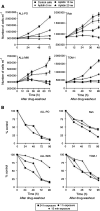
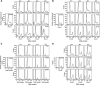
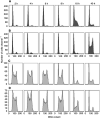
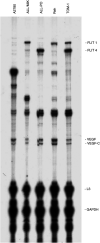
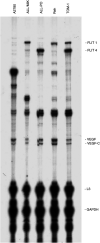
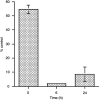
The cytotoxic effect of Aplidin was investigated on fresh leukaemia cells derived from children with B-cell-precursor (BCP) acute lymphoblastic leukaemia (ALL) by using stromal-layer culture system and on four cell lines, ALL-PO, Reh, ALL/MIK and TOM-1, derived from patients with ALL with different molecular genetic abnormalities. In ALL cell lines Aplidin was cytotoxic at nanomolar concentrations. In the ALL cell lines the drug-induced cell death was clearly related to the induction of apoptosis and appeared to be p53-independent. Only in ALL-PO 20 nM Aplidin treatment caused a block of vascular endothelial growth factor (VEGF) secretion and downregulation of VEGF-mRNA, but Aplidin cytotoxicity does not seem to be related to VEGF inhibition since the sensitivity of ALL-PO cells to Aplidin is comparable to that observed for the other cells used. Aplidin induced a G(1) and a G(2) M block in ALL cell lines. In patient-derived leukaemia cells, Aplidin induced a strong cytotoxicity evidenced in a stroma-supported immunocytometric assay. Cells from children with genetic abnormalities such as t(9;22) and t(4;11) translocations, associated with an inferior treatment outcome, were sensitive to Aplidin to the same extent as that observed in other BCP-ALL cases. Aplidin exerted a strong cell killing effect (>88%) against primary culture cells from five relapsed ALL cases, at concentrations much lower than those reported to be achieved in plasma of patients receiving Aplidin at recommended doses. Taken together these data suggest that Aplidin could be a new anticancer drug to be investigated in ALL patients resistant to available therapy.
Figures




References
-
- Albella B, Faircloth G, Lopez-Lazaro L, Guzman C, Jimeno J, Bueren JA (2002) In vitro toxicity of ET-743 and aplidine, two marine-derived antineoplastics, on human bone marrow haematopoietic progenitors, comparison with the clinical results. Eur J Cancer 38: 1395–1404 - PubMed
-
- Armand JP, Ady-Vago N, Faivre S, Chieze S, Baudin E, Ribrag V, Lecot F, Iglesias L, Lopez-Lazaro L, Guzman C, Jimeno J, Ducreux M, Le Chevalier T, Raymond E (2001) Phase I and pharmacokinetic study of aplidine (APL) given as a 24-hour continuous infusion every other week (q2w) in patients (pts) with solid tumor (ST) and lymphoma (NHL)[abstract]. Proceedings 37th ASCO Annual Meeting. San Francisco 12–15 May, Vol. 20, p 120a
-
- Broggini M, Marchini S, Galliera E, Borsotti P, Taraboletti G, Erba E, Sironi M, Giavazzi R, Jimeno J, Faircloth G, D'Incalci M (2003) Aplidine, a new anticancer agent of marine origin, inhibits vascular endothelial growth factor (VEGF) secretion and blocks VEGF-VEGF-RI (flt-1) autocrine loop in human leukemic cells MOLT-4. Leukemia 17: 52–59 - PubMed
-
- Broggini M, Marchini S, Galliera E, D'Incalci M, Taraboletti G, Giavazzi R, Faircloth GT, Jimeno J (2001) Aplidine-induced apoptosis in MOLT-4 cells is mediated by its ability to block VEGF secretion [abstract]. Proceedings AACR- NCI-EORTC International Conference: Molecular targets and cancer therapeutics, Miami, 22 October–2 November, p 79
-
- Campana D, Houghton PJ, Rivera GK (1999) Testing antileukemic drugs. In Childhood Leukemias, Ching-Hong Pui (ed) pp 393–412. Cambridge
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

