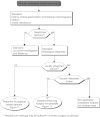
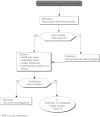
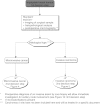
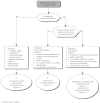
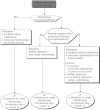
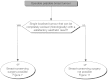
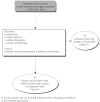
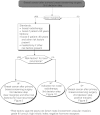
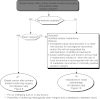
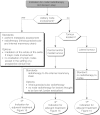
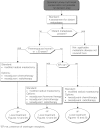
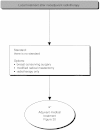
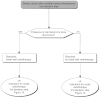
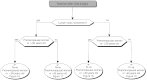
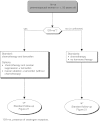
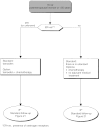
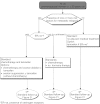
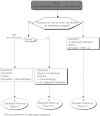
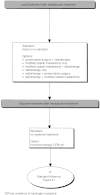
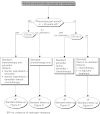
Summary version of the Standards, Options and Recommendations for nonmetastatic breast cancer (updated January 2001)
- PMID: 12915900
- PMCID: PMC2753009
- DOI: 10.1038/sj.bjc.6601081
Summary version of the Standards, Options and Recommendations for nonmetastatic breast cancer (updated January 2001)
Figures
Similar articles
-
[Standards, Options and Recommendations for non metastatic breast cancer patients].Bull Cancer. 2002 Feb;89(2):207-24. Bull Cancer. 2002. PMID: 11888860 French.
-
[Standards, options and recommendations for the management of patients with infiltrating non metastatic breast cancer (2nd edition, 2001)--summary version].Gynecol Obstet Fertil. 2003 Mar;31(3):284-315. doi: 10.1016/s1297-9589(03)00048-1. Gynecol Obstet Fertil. 2003. PMID: 12846249 French. No abstract available.
-
[1st revision of the National Consensus on Breast Cancer Treatment].Ginecol Obstet Mex. 2002 Jul;70:349-60. Ginecol Obstet Mex. 2002. PMID: 12221911 Review. Spanish. No abstract available.
-
Invasive breast cancer.J Natl Compr Canc Netw. 2007 Mar;5(3):246-312. doi: 10.6004/jnccn.2007.0025. J Natl Compr Canc Netw. 2007. PMID: 17439758 Review. No abstract available.
-
Multidisciplinary considerations in the treatment of triple-negative breast cancer.CA Cancer J Clin. 2020 Nov;70(6):432-442. doi: 10.3322/caac.21643. Epub 2020 Sep 28. CA Cancer J Clin. 2020. PMID: 32986241 No abstract available.
Cited by
-
Results of preoperative lymphoscintigraphy for breast cancer are predictive of identification of axillary sentinel lymph nodes.World J Surg. 2006 Jan;30(1):55-62. doi: 10.1007/s00268-005-0145-3. World J Surg. 2006. PMID: 16369717
-
An analysis of the association between cancer-related information seeking and adherence to breast cancer surveillance procedures.Cancer Epidemiol Biomarkers Prev. 2013 Jan;22(1):167-74. doi: 10.1158/1055-9965.EPI-12-0781. Epub 2012 Nov 1. Cancer Epidemiol Biomarkers Prev. 2013. PMID: 23118144 Free PMC article.
-
Development and feasibility of a set of quality indicators relative to the timeliness and organisation of care for new breast cancer patients undergoing surgery.BMC Health Serv Res. 2012 Jun 21;12:167. doi: 10.1186/1472-6963-12-167. BMC Health Serv Res. 2012. PMID: 22721001 Free PMC article.
-
Management and outcomes of male breast cancer in zaria, Nigeria.Int J Breast Cancer. 2012;2012:845143. doi: 10.1155/2012/845143. Epub 2012 Sep 6. Int J Breast Cancer. 2012. PMID: 22991670 Free PMC article.
References
-
- Mauriac L, Asselain B, Blanc-Vincent MP, Cutuli B, Dilhuydy JM, Fourquet A, Garbay JR, Luporsi E, Spyratos F, Zafrani B (1996) Fédération Nationale des Centres de Lutte Center le Cancer, ed. Cancers du sein non métastatiques, Vol. 3. Paris: Arnette Blackwell; Standards Options et Recommandations
-
- Mauriac L, Luporsi E, Cutuli B, Fourquet A, Garbay JR, Giard S, Spyratos F, Sigal-Zafrani B, Dilhuydy JM, Acharian V, Balu-Maestro C, Blanc-Vincent MP, Cohen-Solal C, De Lafontan B, Dilhuydy MH, Duquesne B, Gilles R, Lesur A, Shen N (2001) Fédération Nationale des Centres de Lutte Contre le Cancer, ed. Cancers du sein infiltrants non métastatiques, Vol 12. Paris: John Libbey EUROTEXT; Standards Options & Recommandations
-
- Mauriac L, Luporsi E, Cutuli B, Fourquet A, Garbay JR, Giard S, Spyratos F, Sigal-Zafrani B, Dilhuydy JM, Acharian V, Balu-Maestro C, Blanc-Vincent MP, Cohen-Solal C, De Lafontan B, Dilhuydy MH, Duquesne B, Gilles R, Lesur A, Shen N (2002) Standards, options and recommendations for non metastatic breast cancer patients. Bull Cancer 89: 207–224 - PubMed
-
- Recommandation du comité d’experts de la Société Française de Radiothérapie Oncologique (SFRO) (1991) Quels volumes cibles ganglionnaires irradiés dans le traitement conservateur du cancer du sein T1-T2≤3 cm N0-n1 (UICC 1987). Bull Cancer Radiother 78: 115–117
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

