Of mice and MEN1: Insulinomas in a conditional mouse knockout
- PMID: 12917331
- PMCID: PMC180910
- DOI: 10.1128/MCB.23.17.6075-6085.2003
Of mice and MEN1: Insulinomas in a conditional mouse knockout
Abstract
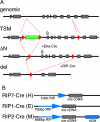
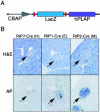
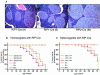
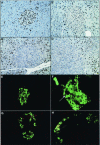
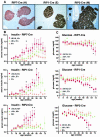
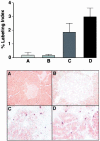
Patients with multiple endocrine neoplasia type 1 (MEN1) develop multiple endocrine tumors, primarily affecting the parathyroid, pituitary, and endocrine pancreas, due to the inactivation of the MEN1 gene. A conditional mouse model was developed to evaluate the loss of the mouse homolog, Men1, in the pancreatic beta cell. Men1 in these mice contains exons 3 to 8 flanked by loxP sites, such that, when the mice are crossed to transgenic mice expressing cre from the rat insulin promoter (RIP-cre), exons 3 to 8 are deleted in beta cells. By 60 weeks of age, >80% of mice homozygous for the floxed Men1 gene and expressing RIP-cre develop multiple pancreatic islet adenomas. The formation of adenomas results in elevated serum insulin levels and decreased blood glucose levels. The delay in tumor appearance, even with early loss of both copies of Men1, implies that additional somatic events are required for adenoma formation in beta cells. Comparative genomic hybridization of beta cell tumor DNA from these mice reveals duplication of chromosome 11, potentially revealing regions of interest with respect to tumorigenesis.
Figures


References
-
- Agarwal, S. K., L. V. Debelenko, M. B. Kester, S. C. Guru, P. Manickam, S. E. Olufemi, M. C. Skarulis, C. Heppner, J. S. Crabtree, I. A. Lubensky, Z. Zhuang, Y. S. Kim, S. C. Chandrasekharappa, F. S. Collins, L. A. Liotta, A. M. Spiegel, A. L. Burns, M. R. Emmert-Buck, and S. J. Marx. 1998. Analysis of recurrent germline mutations in the MEN1 gene encountered in apparently unrelated families. Hum. Mutat. 12:75-82. - PubMed
-
- Agarwal, S. K., S. C. Guru, C. Heppner, M. R. Erdos, R. M. Collins, S. Y. Park, S. Saggar, S. C. Chandrasekharappa, F. S. Collins, A. M. Spiegel, S. J. Marx, and A. L. Burns. 1999. Menin interacts with the AP1 transcription factor JunD and represses JunD-activated transcription. Cell 96:143-152. - PubMed
-
- Bassett, J. H., S. A. Forbes, A. A. Pannett, S. E. Lloyd, P. T. Christie, C. Wooding, B. Harding, G. M. Besser, C. R. Edwards, J. P. Monson, J. Sampson, J. A. Wass, M. H. Wheeler, and R. V. Thakker. 1998. Characterization of mutations in patients with multiple endocrine neoplasia type 1. Am. J. Hum Genet. 62:232-244. - PMC - PubMed
-
- Chandrasekharappa, S. C., S. C. Guru, P. Manickam, S. E. Olufemi, F. S. Collins, M. R. Emmert-Buck, L. V. Debelenko, Z. Zhuang, I. A. Lubensky, L. A. Liotta, J. S. Crabtree, Y. Wang, B. A. Roe, J. Weisemann, M. S. Boguski, S. K. Agarwal, M. B. Kester, Y. S. Kim, C. Heppner, Q. Dong, A. M. Spiegel, A. L. Burns, and S. J. Marx. 1997. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science 276:404-407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
