USF1 and USF2 mediate inhibition of human trophoblast differentiation and CYP19 gene expression by Mash-2 and hypoxia
- PMID: 12917334
- PMCID: PMC180905
- DOI: 10.1128/MCB.23.17.6117-6128.2003
USF1 and USF2 mediate inhibition of human trophoblast differentiation and CYP19 gene expression by Mash-2 and hypoxia
Abstract
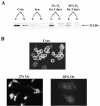
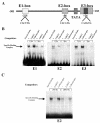
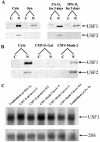
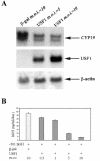
In the human placental syncytiotrophoblast, C(19) steroids are converted to estrogens by aromatase P450, product of the CYP19 gene. When human cytotrophoblasts, which lack the capacity to express aromatase, are cultured in 20% O(2), they spontaneously fuse to form a multinuclear syncytiotrophoblast and CYP19 expression is markedly induced. On the other hand, when cytotrophoblasts are cultured in 2% O(2), syncytiotrophoblast differentiation and induction of CYP19 expression are prevented. We previously observed that expression of the transcription factor Mash-2 (mammalian achaete/scute homologue 2), which is elevated in human cytotrophoblasts and maintained at elevated levels by hypoxia, declines with syncytiotrophoblast differentiation. Overexpression of Mash-2 prevents syncytiotrophoblast differentiation and induction of CYP19 expression. In the present study, we observed that unexpectedly immunoreactive Mash-2 protein was localized predominantly to the cytoplasm of human cytotrophoblasts. Elevated cytoplasmic levels of Mash-2 were maintained when trophoblasts were cultured in 2% O(2) and declined to undetectable levels upon culture in 20% O(2). Previously, we found that Mash-2 inhibited CYP19 promoter activity through sequences within a 350-bp region upstream and within placenta-specific exon I.1 containing three E boxes (E1 at -325 bp, 5'-CACTTG-3'; E2 at -58 bp, 5'-CACATG-3'; and E3 at +26 bp, 5'-CACGTG-3'). In this study, we found that trophoblast nuclear protein binding to these E boxes declined with syncytiotrophoblast differentiation in 20% O(2) and was induced by hypoxia; however, Mash-2 did not appear to bind to any of these E boxes. On the other hand, the basic helix-loop-helix leucine zipper transcription factors upstream stimulatory factors 1 and 2 (USF1 and USF2) did bind to E2 and E3 but not E1. Nuclear levels of USF1 and USF2 and DNA-binding activity declined with syncytiotrophoblast differentiation and were maintained at elevated levels by hypoxia and overexpression of Mash-2, whereas USF1 mRNA levels were unaffected. Finally, USF1 overexpression in cultured human trophoblasts markedly inhibited endogenous CYP19 expression, differentiation of cultured human trophoblast cells, and CYP19 promoter activity. These findings suggest that increased protein levels and DNA binding of USF1 and USF2 mediate the inhibitory effects of hypoxia and of Mash-2 on CYP19 gene expression in human placenta.
Figures
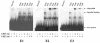


Similar articles
-
Hypoxia prevents induction of aromatase expression in human trophoblast cells in culture: potential inhibitory role of the hypoxia-inducible transcription factor Mash-2 (mammalian achaete-scute homologous protein-2).Mol Endocrinol. 2000 Oct;14(10):1661-73. doi: 10.1210/mend.14.10.0539. Mol Endocrinol. 2000. PMID: 11043580
-
O2 enhancement of human trophoblast differentiation and hCYP19 (aromatase) gene expression are mediated by proteasomal degradation of USF1 and USF2.Mol Cell Biol. 2005 Oct;25(20):8824-33. doi: 10.1128/MCB.25.20.8824-8833.2005. Mol Cell Biol. 2005. PMID: 16199862 Free PMC article.
-
Characterization of the regulatory regions of the human aromatase (P450arom) gene involved in placenta-specific expression.Mol Endocrinol. 1998 Nov;12(11):1764-77. doi: 10.1210/mend.12.11.0190. Mol Endocrinol. 1998. PMID: 9817601
-
Transcription factors underlying the development and endocrine functions of the placenta.Recent Prog Horm Res. 2002;57:221-34. doi: 10.1210/rp.57.1.221. Recent Prog Horm Res. 2002. PMID: 12017545 Review.
-
The regulation of trophoblast differentiation by oxygen in the first trimester of pregnancy.Hum Reprod Update. 2006 Mar-Apr;12(2):137-44. doi: 10.1093/humupd/dmi043. Epub 2005 Oct 18. Hum Reprod Update. 2006. PMID: 16234296 Review.
Cited by
-
OVO-like 1 regulates progenitor cell fate in human trophoblast development.Proc Natl Acad Sci U S A. 2015 Nov 10;112(45):E6175-84. doi: 10.1073/pnas.1507397112. Epub 2015 Oct 26. Proc Natl Acad Sci U S A. 2015. PMID: 26504231 Free PMC article.
-
Estrogen receptor alpha (ERalpha) mediates stimulatory effects of estrogen on aromatase (CYP19) gene expression in human placenta.Mol Endocrinol. 2009 Jun;23(6):784-93. doi: 10.1210/me.2008-0371. Epub 2009 Mar 19. Mol Endocrinol. 2009. PMID: 19299445 Free PMC article.
-
Human prolyl-4-hydroxylase alpha(I) transcription is mediated by upstream stimulatory factors.J Biol Chem. 2006 Apr 21;281(16):10849-55. doi: 10.1074/jbc.M511237200. Epub 2006 Feb 17. J Biol Chem. 2006. PMID: 16488890 Free PMC article.
-
Upstream stimulating factors 1 and 2 enhance transcription from the placenta-specific promoter 1.1 of the bovine cyp19 gene.BMC Mol Biol. 2010 Jan 18;11:5. doi: 10.1186/1471-2199-11-5. BMC Mol Biol. 2010. PMID: 20082704 Free PMC article.
-
The c-Myc-regulated microRNA-17~92 (miR-17~92) and miR-106a~363 clusters target hCYP19A1 and hGCM1 to inhibit human trophoblast differentiation.Mol Cell Biol. 2013 May;33(9):1782-96. doi: 10.1128/MCB.01228-12. Epub 2013 Feb 25. Mol Cell Biol. 2013. PMID: 23438603 Free PMC article.
References
-
- Alcorn, J. L., M. E. Smith, J. F. Smith, L. R. Margraf, and C. R. Mendelson. 1997. Primary cell culture of human type II pneumonocytes: maintenance of a differentiated phenotype and transfection with recombinant adenoviruses. Am. J. Respir. Cell Mol. Biol. 17:672-682. - PubMed
-
- Alsat, E., P. Wyplosz, A. Malassine, J. Guibourdenche, D. Porquet, C. Nessmann, and D. Evain-Brion. 1996. Hypoxia impairs cell fusion and differentiation process in human cytotrophoblast, in vitro. J. Cell. Physiol. 168:346-353. - PubMed
-
- Boggaram, V., K. Qing, and C. R. Mendelson. 1988. The major apoprotein of rabbit pulmonary surfactant. Elucidation of primary sequence and cyclic AMP and developmental regulation. J. Biol. Chem. 263:2939-2947. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
