ZIP kinase triggers apoptosis from nuclear PML oncogenic domains
- PMID: 12917339
- PMCID: PMC180930
- DOI: 10.1128/MCB.23.17.6174-6186.2003
ZIP kinase triggers apoptosis from nuclear PML oncogenic domains
Abstract
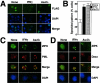
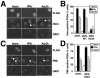
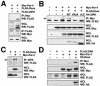
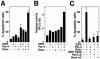
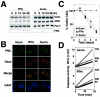
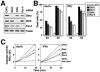
PML oncogenic domains (PODs), also referred to as nuclear dot 10 bodies, Kreb's bodies, or nuclear bodies, represent nuclear structures implicated in the regulation of a variety of cellular processes, including transcription, tumor suppression, and apoptosis. ZIP kinase (ZIPK) is a proapoptotic protein kinase with homology to DAP kinase, a protein kinase implicated in apoptosis. We show here that ZIPK is present in PODs, where it colocalizes with and binds to proapoptotic protein Daxx. Arsenic trioxide (As(2)O(3)) and gamma interferon (IFN-gamma), which accentuate POD formation, increased the association of ZIPK with PODs. In contrast, the kinase-inactive ZIPK resides in nuclei with a diffuse pattern and significantly prevents the association of Daxx with PODs, implying that ZIPK recruits Daxx to PODs via its catalytic activity. ZIPK also binds and phosphorylates proapoptotic protein Par-4. Association of ZIPK with Daxx was enhanced by coexpression of Par-4. Activation of caspases and induction of apoptosis were also observed in cells overexpressing these proteins. Conversely, small-interfering RNA-mediated reduction of ZIPK, Daxx, or Par-4 expression decreased activation of caspase and apoptosis induced by As(2)O(3) and IFN-gamma. These results suggest that ZIPK, in collaboration with Daxx and Par-4, mediates a novel nuclear pathway for apoptosis.
Figures



Similar articles
-
Human Daxx regulates Fas-induced apoptosis from nuclear PML oncogenic domains (PODs).EMBO J. 1999 Nov 1;18(21):6037-49. doi: 10.1093/emboj/18.21.6037. EMBO J. 1999. PMID: 10545115 Free PMC article.
-
Promyelocytic leukemia protein (PML) functions as a glucocorticoid receptor co-activator by sequestering Daxx to the PML oncogenic domains (PODs) to enhance its transactivation potential.J Biol Chem. 2003 May 2;278(18):15958-65. doi: 10.1074/jbc.M300387200. Epub 2003 Feb 20. J Biol Chem. 2003. PMID: 12595526
-
Daxx silencing sensitizes cells to multiple apoptotic pathways.Mol Cell Biol. 2003 Oct;23(20):7108-21. doi: 10.1128/MCB.23.20.7108-7121.2003. Mol Cell Biol. 2003. PMID: 14517282 Free PMC article.
-
PML nuclear bodies and apoptosis.Oncogene. 2004 Apr 12;23(16):2819-24. doi: 10.1038/sj.onc.1207533. Oncogene. 2004. PMID: 15077145 Review.
-
PML NBs (ND10) and Daxx: from nuclear structure to protein function.Front Biosci. 2008 May 1;13:7132-42. doi: 10.2741/3216. Front Biosci. 2008. PMID: 18508722 Review.
Cited by
-
Prostate apoptosis response 4 (Par-4), a novel substrate of caspase-3 during apoptosis activation.Mol Cell Biol. 2012 Feb;32(4):826-39. doi: 10.1128/MCB.06321-11. Epub 2011 Dec 19. Mol Cell Biol. 2012. PMID: 22184067 Free PMC article.
-
Mapping the chromosomal targets of STAT1 by Sequence Tag Analysis of Genomic Enrichment (STAGE).Genome Res. 2007 Jun;17(6):910-6. doi: 10.1101/gr.5574907. Genome Res. 2007. PMID: 17568006 Free PMC article.
-
Zipper-interacting protein kinase (ZIPK) modulates canonical Wnt/beta-catenin signaling through interaction with Nemo-like kinase and T-cell factor 4 (NLK/TCF4).J Biol Chem. 2011 May 27;286(21):19170-7. doi: 10.1074/jbc.M110.189829. Epub 2011 Mar 30. J Biol Chem. 2011. PMID: 21454679 Free PMC article.
-
Identification and functional significance of genes regulated by structurally different histone deacetylase inhibitors.Proc Natl Acad Sci U S A. 2005 Mar 8;102(10):3697-702. doi: 10.1073/pnas.0500369102. Epub 2005 Feb 28. Proc Natl Acad Sci U S A. 2005. PMID: 15738394 Free PMC article.
-
ZIPK: a unique case of murine-specific divergence of a conserved vertebrate gene.PLoS Genet. 2007 Oct;3(10):1884-93. doi: 10.1371/journal.pgen.0030180. Epub 2007 Sep 7. PLoS Genet. 2007. PMID: 17953487 Free PMC article.
References
-
- Camandola, S., and M. P. Mattson. 2000. Pro-apoptotic action of PAR-4 involves inhibition of NF-κB activity and suppression of BCL-2 expression. J. Neurosci. Res. 61:134-139. - PubMed
-
- Cardone, M. H., N. Roy, H. R. Stennicke, G. S. Salvesen, T. F. Franke, E. Stanbridge, S. Frisch, and J. C. Reed. 1998. Regulation of cell death protease caspase-9 by phosphorylation. Science 282:1318-1320. - PubMed
-
- Chang, H., H. Nishitoh, X. Yang, H. Ichijo, and D. Baltimore. 1998. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein daxx. Science 281:1860-1863. - PubMed
-
- Cohen, O., and A. Kimchi 2001. DAP-kinase: from functional gene cloning to establishment of its role in apoptosis and cancer. Cell Death Differ. 8:6-15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
