Vav1 dephosphorylation by the tyrosine phosphatase SHP-1 as a mechanism for inhibition of cellular cytotoxicity
- PMID: 12917349
- PMCID: PMC180957
- DOI: 10.1128/MCB.23.17.6291-6299.2003
Vav1 dephosphorylation by the tyrosine phosphatase SHP-1 as a mechanism for inhibition of cellular cytotoxicity
Abstract
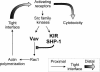
Here, we present data suggesting a novel mechanism for regulation of natural killer (NK) cell cytotoxicity through inhibitory receptors. Interaction of activation receptors with their ligands on target cells induces cytotoxicity by NK cells. This activation is under negative control by inhibitory receptors that recruit tyrosine phosphatase SHP-1 upon binding major histocompatibility class I on target cells. How SHP-1 blocks the activation pathway is not known. To identify SHP-1 substrates, an HLA-C-specific inhibitory receptor fused to a substrate-trapping mutant of SHP-1 was expressed in NK cells. Phosphorylated Vav1, a regulator of actin cytoskeleton, was the only protein detectably associated with the catalytic site of SHP-1 during NK cell contact with target cells expressing HLA-C. Vav1 trapping was independent of actin polymerization, suggesting that inhibition of cellular cytotoxicity occurs through an early dephosphorylation of Vav1 by SHP-1, which blocks actin-dependent activation signals. Such a mechanism explains how inhibitory receptors can block activating signals induced by different receptors.
Figures

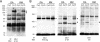

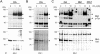


References
-
- Aghazadeh, B., W. E. Lowry, X. Y. Huang, and M. K. Rosen. 2000. Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell 102:625-633. - PubMed
-
- Barford, D., and B. G. Neel. 1998. Revealing mechanisms for SH2 domain mediated regulation of the protein tyrosine phosphatase SHP-2. Structure 6:249-254. - PubMed
-
- Bauer, S., V. Groh, J. Wu, A. Steinle, J. H. Phillips, L. L. Lanier, and T. Spies. 1999. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285:727-729. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
