A novel domain in adenovirus L4-100K is required for stable binding and efficient inhibition of human granzyme B: possible interaction with a species-specific exosite
- PMID: 12917351
- PMCID: PMC180958
- DOI: 10.1128/MCB.23.17.6315-6326.2003
A novel domain in adenovirus L4-100K is required for stable binding and efficient inhibition of human granzyme B: possible interaction with a species-specific exosite
Abstract
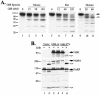
Lymphocyte granule serine proteases (granzymes) play a critical role in protecting higher organisms against intracellular infections and cellular transformation. The proteases have also been implicated in the generation of tissue damage in a variety of chronic human conditions, including autoimmunity and transplant rejection. Granzyme B (GrB), one cytotoxic member of this family, achieves its effect through cleavage and activation of caspases as well as through caspase-independent proteolysis of cellular substrates. The 100,000-molecular-weight (100K) assembly protein of human adenovirus type 5 (Ad5-100K) was previously defined as a potent and specific inhibitor of human GrB. We now show that although human, mouse, and rat GrB proteases are well conserved in terms of structure, substrate specificity, and function, Ad5-100K inhibitory activity is directed exclusively against the human protease. Biochemical analysis demonstrates that the specificity of the 100K protein for human GrB resides in two distinct interactions with the protease: (i) a unique sequence within the reactive site loop (P(1))Asp(48)-(P(1'))Pro(49) in Ad5-100K which interacts with the active site and (ii) the presence of an additional inhibitor-enzyme interaction likely outside the enzyme catalytic site (i.e., an exosite). We have located this extended macromolecular interaction site in Ad5-100K within amino acids 688 to 781, and we have demonstrated that this region is essential for stable inhibitor-enzyme complex formation as well as efficient inhibition of human GrB. This novel component of the inhibitory mechanism of the 100K protein identifies a distinct target for selective inhibitor design, a finding which may be of benefit for diseases in which GrB plays a pathogenic role.
Figures
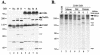

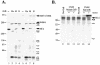



References
-
- Alimonti, J. B., L. Shi, P. K. Baijal, and A. H. Greenberg. 2001. Granzyme B induces BID-mediated cytochrome c release and mitochondrial permeability transition. J. Biol. Chem. 276:6974-6982. - PubMed
-
- Andrade, F., H. G. Bull, N. A. Thornberry, G. W. Ketner, L. A. Casciola-Rosen, and A. Rosen. 2001. Adenovirus L4-100K assembly protein is a granzyme B substrate that potently inhibits granzyme B-mediated cell death. Immunity 14:751-761. - PubMed
-
- Andrade, F., L. Casciola-Rosen, and A. Rosen. 2000. Apoptosis in systemic lupus erythematosus. Clinical implications. Rheum. Dis. Clin. North Am. 26:215-227. - PubMed
-
- Andrade, F., S. Roy, D. Nicholson, N. Thornberry, A. Rosen, and L. Casciola-Rosen. 1998. Granzyme B directly and efficiently cleaves several downstream caspase substrates: implications for CTL-induced apoptosis. Immunity 8:451-460. - PubMed
-
- Ashkenazi, A., and V. M. Dixit. 1998. Death receptors: signaling and modulation. Science 281:1305-1308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
