Presynaptic mechanism for anti-analgesic and anti-hyperalgesic actions of kappa-opioid receptors
- PMID: 12917359
- PMCID: PMC6740440
- DOI: 10.1523/JNEUROSCI.23-19-07262.2003
Presynaptic mechanism for anti-analgesic and anti-hyperalgesic actions of kappa-opioid receptors
Abstract
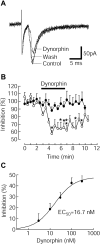
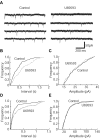
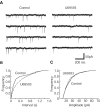
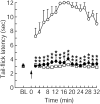
Glutamate neurotransmission plays an important role in the processing of pain and in chronic opioid-induced neural and behavioral plasticity, such as opioid withdrawal and opioid dependence. Kappa-opioid receptors also have been implicated in acute opioid modulation of pain and chronic opioid-induced plasticity, both of which are primarily mediated by mu-opioid receptors. Using whole-cell patch clamp recordings in brain slices in vitro and system analysis of pain behaviors in rats in vivo, this study investigated the functional role of glutamate synaptic transmission and kappa-opioid receptors in two behavioral pain conditions: m-opioid-induced analgesia (decreased pain) and mu-opioid withdrawal-induced hyperalgesia (increased pain). In the nucleus raphe magnus (NRM), a brainstem structure that controls spinal pain transmission, we found that kappa-receptor agonists presynaptically inhibited glutamate synaptic currents in both of the two cell types that are thought to respectively inhibit or facilitate spinal pain transmission. In rats, both glutamate receptor antagonists and the kappa agonist microinjected into the NRM attenuated mu-opioid-induced analgesia, which is most likely mediated through activation of such pain-inhibiting neurons. However, during opioid abstinence-induced withdrawal, the same doses of glutamate receptor antagonists and the kappa agonist administered in the NRM suppressed the withdrawal-induced hyperalgesia, which is thought to be mediated by activation of those pain-facilitating neurons during opioid withdrawal. These results demonstrate that kappa-opioid receptors antagonize mu-receptor-induced effects in both analgesic and hyperalgesic states, and suggest inhibition of glutamate synaptic transmission as a presynaptic mechanism for the kappa antagonism of these two mu receptor-mediated actions.
Figures


References
-
- Ackley MA, Hurley RW, Virnich DE, Hammond DL ( 2001) A cellular mechanism for the antinociceptive effect of a kappa opioid receptor agonist. Pain 91: 377-388. - PubMed
-
- Cheng HY, Pitcher GM, Laviolette SR, Whishaw IQ, Tong KI, Kockeritz LK, Wada T, Joza NA, Crackower M, Goncalves J, Sarosi I, Woodgett JR, Oliveira-dos-Santos AJ, Ikura M, van der Kooy D, Salter MW, Penninger JM ( 2002) DREAM is a critical transcriptional repressor for pain modulation. Cell 108: 31-43. - PubMed
-
- Coderre TJ, Katz J, Vaccarino AL, Melzack R ( 1993) Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain 52: 259-285. - PubMed
-
- Fields H, Basbaum A ( 1999) Central nervous system mechanisms of pain modulation. In: Textbook of pain, 4E Ed (Wall P, Melzack R, eds). London: Churchill Livingstone.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
