Hypertrophic neuropathies and malignant peripheral nerve sheath tumors in transgenic mice overexpressing glial growth factor beta3 in myelinating Schwann cells
- PMID: 12917360
- PMCID: PMC6740435
- DOI: 10.1523/JNEUROSCI.23-19-07269.2003
Hypertrophic neuropathies and malignant peripheral nerve sheath tumors in transgenic mice overexpressing glial growth factor beta3 in myelinating Schwann cells
Abstract
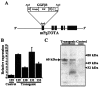
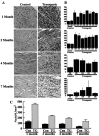
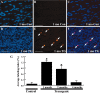
The neuregulin-1 (NRG-1) family of growth and differentiation factors exerts a variety of effects on Schwann cells and their precursors during nervous system development; however, NRG-1 effects on adult Schwann cells are poorly defined. Several lines of evidence suggest that NRG-1 actions on adult Schwann cells are distinct from those observed during development. To test this hypothesis, we generated transgenic mice overexpressing the NRG-1 isoform glial growth factor beta3 (GGFbeta3) in myelinating Schwann cells [protein zero (P0)GGFbeta3 mice]. P0-GGFbeta3 mice develop resting tremors, gait abnormalities, decreased hindlimb strength, and paralysis by approximately 7 months of age. Sciatic nerves from these animals show a hypertrophic neuropathy characterized by demyelination, remyelination, and "onion bulb" formation. Development of this hypertrophic neuropathy is preceded by Schwann cell hyperplasia that is prominent in 1-month-old mice and present but decreased in 2- and 4-month-old animals. P0-GGFbeta3 mice also develop peripheral ganglion-associated malignant peripheral nerve sheath tumors. Motor, sensory, and sympathetic ganglia from 1-, 2-, and 4-month-old P0-GGFbeta3 mice uniformly contain intraganglionic, likely preneoplastic, Schwann cell proliferations. Examination of bromodeoxyuridine incorporation and caspase-3 activation in sciatic nerves and trigeminal ganglia indicates that Schwann cell hyperplasia in P0-GGFbeta3 mice reflects increased proliferation rather than decreased apoptosis. These observations are consistent with the hypothesis that GGFbeta3 induces proliferation of adult Schwann cells and demyelination of peripheral nerve axons. Furthermore, overexpression of this NRG-1 isoform frequently induces neoplastic Schwann cell proliferation within PNS ganglia, suggesting that NRG-1 may contribute to human Schwann cell neoplasia.
Figures





References
-
- Adlkofer K, Lai C ( 2000) Role of neuregulins in glial cell development. Glia 29: 104-111. - PubMed
-
- Anderson LM, Hagiwara A, Kovatch RM, Rehm S, Rice JM ( 1989) Transplacental initiation of liver, lung, neurogenic and connective tissue tumors by N-nitroso compounds in mice. Fund Appl Toxicol 12: 604-620. - PubMed
-
- Arpornchayanon O, Hirota T, Itabashi M, Nakajima T, Fukuma H, Beppu Y, Nishikawa K ( 1984) Malignant peripheral nerve tumors: a clinicopathological and electron microscopic study. Jpn J Clin Oncol 14: 57-74. - PubMed
-
- Baron-Van Evercooren A, Gansmuller A, Gumpel M, Baumann N, Kleinman HK ( 1986) Schwann cell differentiation in vitro: extracellular matrix deposition and interaction. Dev Neurosci 8: 182-196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
