Presynaptic and postsynaptic mechanisms of a novel form of homosynaptic potentiation at aplysia sensory-motor neuron synapses
- PMID: 12917362
- PMCID: PMC6740441
- DOI: 10.1523/JNEUROSCI.23-19-07288.2003
Presynaptic and postsynaptic mechanisms of a novel form of homosynaptic potentiation at aplysia sensory-motor neuron synapses
Abstract
Previous studies have shown that homosynaptic potentiation produced by rather mild tetanic stimulation (20 Hz, 2 sec) at Aplysia sensory-motor neuron synapses in isolated cell culture involves both presynaptic and postsynaptic Ca2+ (Bao et al., 1997). We have now investigated the sources of Ca2+ and some of its downstream targets. Although the potentiation lasts >30 min, it does not require Ca2+ influx through either NMDA receptor channels or L-type Ca2+ channels. Rather, the potentiation involves metabotropic receptors and intracellular Ca2+ release from both postsynaptic IP3-sensitive and presynaptic ryanodine-sensitive stores. In addition, it involves protein kinases, including both presynaptic and postsynaptic CamKII and probably MAP kinase. Finally, it does not require transsynaptic signaling by nitric oxide but it may involve AMPA receptor insertion. The potentiation, thus, shares components of the mechanisms of post-tetanic potentiation, NMDA- and mGluR-dependent long-term potentiation, and even long-term depression, but is not identical to any of them. These results are consistent with the more general idea that there is a molecular alphabet of basic components that can be combined in various ways to create novel as well as known types of plasticity.
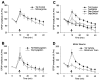
Figures






References
-
- Antonov I, Antonova I, Minnal A, Hawkins RD ( 2001) Possible interaction of pre- and postsynaptic mechanisms during classical conditioning in Aplysia Soc Neurosci Abstr 27: 954.15.
-
- Antonov I, Antonova I, Kandel ER, Hawkins RD ( 2003) Activity-dependent presynaptic facilitation and Hebbian LTP are both required and interact during classical conditioning in Aplysia Neuron 37: 135-147. - PubMed
-
- Anwyl R, Lee WL, Rowan M ( 1988) The role of calcium in short-term potentiation in the rat hippocampal slice. Brain Res 459: 192-195. - PubMed
-
- Bao JX, Kandel ER, Hawkins RD ( 1997) Involvement of pre- and postsynaptic mechanisms in posttetanic potentiation at Aplysia synapses. Science 275: 969-973. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
