Excitatory amino acid antagonists for acute stroke
- PMID: 12917902
- PMCID: PMC7027997
- DOI: 10.1002/14651858.CD001244
Excitatory amino acid antagonists for acute stroke
Abstract
Background: Focal cerebral ischaemia causes release of excitatory amino acid (EAA) neurotransmitters, principally glutamate, with resultant over-stimulation of EAA receptors and downstream pathways. Excess glutamate release is a pivotal event in the evolution of irreversible ischaemic damage in animal models of ischaemia, and drugs that modulate glutamate action either by inhibiting its release, or blocking post-synaptic receptors, are potent neuroprotective agents. Many clinical trials with EAA modulating drugs have been conducted, none individually demonstrating efficacy.
Objectives: To synthesise all the available data on all different classes of EAA modulators and to evaluate evidence of effects on outcome systematically.
Search strategy: Relevant trials were identified in the Specialised Register of Controlled Trials (last searched May 2001). In addition, MEDLINE and EMBASE online searches for the terms "neuroprotection" (and its variants), "neuroprotective agent", for all individual drugs and drug classes included in the review, hand searches of conference proceedings from European, International, American Heart Association and Princeton conferences on Stroke, American Neurological Association and American Academy of Neurology meetings from 1992-2001, and direct contact with individual investigators and pharmaceutical companies.
Selection criteria: Trials were included if they were randomised, controlled studies giving agents with pharmacological properties that included modification of release of EAAs, or blockade of EAA receptors, in stroke within 24h of onset. Efficacy analysis was restricted to trials with a parallel group design: dose escalation studies were excluded. Intention-to-treat analyses were performed on all data. Outcome had to be reported in terms of death or dependence 1-12 months after the acute event.
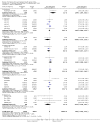
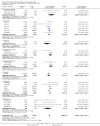
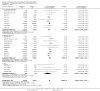
Data collection and analysis: Data were available for 36 of 41 relevant trials identified, involving 11,209 subjects. Data were unavailable for 632 participants (517 in trials fulfilling criteria for efficacy analysis). Seven trials did not report disability data, which were available for 29 trials involving 10,802 subjects. Twenty one of these trials, involving 10,342 subjects, were parallel group studies included in the primary efficacy analysis. Efficacy analysis included data derived from 9 trials not primarily designed to assess efficacy (1022 subjects). The primary (efficacy) end-point was the proportion of patients dead or disabled at final follow-up (defined by Barthel Index<60 at 3 months by preference). Mortality was a secondary end-point. Drugs were considered as individual agents, and also grouped principally into categories of ion channel modulators (glutamate release inhibition) and NMDA antagonists.
Main results: There was no significant heterogeneity of outcome amongst individual drugs, or of drug classes either for the primary efficacy analysis (death or dependence) or for mortality at final follow-up. For the primary efficacy analysis, odds of death or dependence were 1.03 [95% confidence interval 0.96-1.12], and for mortality 1.02 [0.92-1.12]. Neither ion channel modulators (death or dependence 1.02 [0.90-1.16]) nor NMDA antagonists (death or dependence 1.05 [0.95-1.16]) differed from the principal analysis including all compounds. Trends for increased mortality with three NMDA antagonists were seen - selfotel (OR 1.19 [0.81-1.74]), aptiganel (OR 1.32 [0.91-1.93]) and gavestinel (OR 1.12 [0.95-1.32]) - but this did not achieve significance for the NMDA antagonists considered as a class (1.09 [0.96-1.23]). Aptiganel was also associated with a trend towards worse functional outcome (OR 1.20 [0.88-1.65]) although this was not the case for either of the other two compounds. No statistically significant detriment of psychotomimetic NMDA antagonists was found, although a trend towards higher mortality in this sub-group was seen (OR 1.25 [0.96-1.64]).
Reviewer's conclusions: There was no evidence of significant benefit or harm from drugs modulating excitatory amino acid action. Reductio]).
Reviewer's conclusions: There was no evidence of significant benefit or harm from drugs modulating excitatory amino acid action. Reduction of death or dependence by 8% or more has been excluded for gavestinel and lubeluzole, which contribute most of the data for this review. However, mechanistic understanding of neuroprotection is too poor to extrapolate from these two failed development plans to all glutamate modulators. Further clinical trials of neuroprotective agents remain justified, since confidence limits around estimates of effect remain wide for most agents, and cannot reliably exclude benefit. Although numbers of patients are too small to confirm or refute a trend towards increased mortality with some NMDA antagonists, further commercial development of these agents is exceedingly unlikely.
Conflict of interest statement
Prof Lees was principal investigator in GAIN‐International. Both authors are principal investigators in the Intravenous Magnesium Efficacy in Stroke trial, funded by the UK Medical Research Council. Both authors have received fees or expenses for speaking at symposia and / or consultancy on behalf of pharmaceutical companies in the neuroprotective field, including GlaxoWellcome, AstraZeneca, Boehringer Ingelheim, Pfizer, Janssen‐Cilag, Cambridge NeuroScience, and Roche. Both authors have received sponsorship for attendance at meetings and symposia organised by these companies and received payments for speaking at events related to neuroprotection.
Figures








References
References to studies included in this review
AR‐R15896AR {published and unpublished data}
-
- Lees KR, Dyker AG, Sharma A, Ford GA, Ardron ME, Grosset DG. Tolerability of the low‐affinity, use‐dependent NMDA antagonist AR‐R15896AR in stroke patients: a dose‐ranging study. Stroke 2001;32:466‐472. - PubMed
-
- Lees KR, Dyker AG, Sharma A, Ford GA, Ardron ME, Grosset DG, Dean A. AR‐R15896AR in patients with acute stroke: a dose‐finding study. Cerebrovascular Diseases 1999;9(Supplement 1):102.
ASSIST Protocols 7 & 10 {published and unpublished data}
-
- Davis SM, Albers GW, Diener HC, Lees KR, Norris J. Termination of Acute Stroke Studies Involving Selfotel Treatment. ASSIST Steering Committee. Lancet 1997;349:32. - PubMed
CNS1102‐003 {published data only}
-
- Block GA. Final results from a dose‐escalating safety and tolerance study of the non‐competitive NMDA antagonist CNS1102 in patients with acute cerebral ischemia. Stroke 1995;26:185.
-
- Fisher M. Cerestat (CNS 1102), a non‐competitive NMDA antagonist, in ischemic stroke patients: dose‐escalating safety study. Cerebrovascular Diseases 1994;4:245.
-
- McBurney RN. Development of the NMDA ion‐channel blocker, aptiganel hydrochloride, as a neuroprotective agent for acute CNS injury. In: Green AR, Cross AJ editor(s). Neuroprotective Agents and Cerebral Ischaemia. Vol. 40, San Diego: Academic Press, 1997:173‐195. [0‐12‐366840‐9] - PubMed
CNS1102‐008 {unpublished data only}
-
- Edwards K. Cerestat (aptiganel hydrochloride) in the treatment of acute ischemic stroke: results of a phase II trial. Neurology 1996;46(Supplement 2):A424.
CNS1102‐010 {unpublished data only}
-
- Dyker AG, Edwards KR, Fayad PB, Hormes JT, Lees KR. Safety and Tolerability Study of Aptiganel Hydrochloride in Patients With an Acute Ischemic Stroke. Stroke 1999;30(10):2038‐2042. - PubMed
CNS1102‐011 {published data only}
-
- CNS 1102‐011 Trial Synopsis. Unpublished 1997.
-
- Albers GW, Goldstein LB, Hall D, Lesko LM. Aptiganel hydrochloride in acute ischemic stroke: a randomized controlled trial. JAMA 2001;286(21):2673‐2682. - PubMed
CVD 715 {unpublished data only}
-
- Depierre C. Clinical evaluation of eliprodil in the acute phase of stroke. Sanofi‐Synthelabo Report 2000.
Dextrorphan {published data only}
-
- Albers GW, Atkinson RP, Kelley RE, Rosenbaum DM. Safety, tolerability, and pharmacokinetics of the N‐methyl‐D‐ aspartate antagonist dextrorphan in patients with acute stroke. Stroke 1995;26:254‐258. - PubMed
Eliprodil {unpublished data only}
-
- Depierre C. Clinical evaluation of eliprodil in the acute phase of stroke. Sanofi‐Synthelabo report 2000.
Fosphenytoin IIa {published data only}
-
- Tietjen GE, Dombi T, Pulsinelli W, Becske T, Kugler AR, Mann ME. A double‐blind, safety, and tolerance study of single intravenous doses of fosphenytoin in patients with acute ischemic stroke. Neurology 1996;46(Supplement 2):A424‐A425.
GAIN ICH {published data only}
-
- Davis S, Zafar K, Vohora S, Okubo S, Nagashima J, Ito T, Katayama Y. Efficacy and safety of GV150526 in primary intracerebral haemorrhage: data from the GAIN trials (Glycine Antagonist in Neuroprotection). Stroke 2000;31(11):2865.
GAIN‐A {published data only}
-
- Sacco RL. GV150526 in the treatment of acute stroke: results of the GAIN Americas trial. Cerebrovascular Diseases. 2001; Vol. 10, issue Supplement 2:106.
-
- Sacco RL, DeRosa JT, Haley EC, Jr, Levin B, Ordronneau P, Phillips SJ, et al. Glycine antagonist in neuroprotection for patients with acute stroke: GAIN Americas: a randomized controlled trial. JAMA 2001;285(13):1719‐1728. - PubMed
GAIN‐I {published data only}
-
- Lees KR, Asplund K, Carolei A, Davis SM, Diener HC, Orgogozo JM, Whitehead J. Glycine antagonist (gavestinel) in neuroprotection (GAIN International) in patients with acute stroke: a randomised controlled trial. Lancet 2000;355:1949‐1954. - PubMed
GLYA2001 {unpublished data only}
-
- Phase II studies of the glycine antagonist GV150526 in acute stroke : the North American experience. The North American Glycine Antagonist in Neuroprotection (GAIN) Investigators. Stroke 2000;31(2):358‐365. - PubMed
GLYA2005 {unpublished data only}
-
- Phase II studies of the glycine antagonist GV150526 in acute stroke : the North American experience. The North American Glycine Antagonist in Neuroprotection (GAIN) Investigators. Stroke 2000;31(2):358‐365. - PubMed
GLYB2001 {unpublished data only}
-
- Dyker AG, Lees KR. Safety and tolerability of GV150526 (a glycine site antagonist at the N‐ methyl‐D‐aspartate receptor) in patients with acute stroke. Stroke 1999;30(5):986‐992. - PubMed
GLYB2002 {unpublished data only}
-
- GAIN European Study Group. Safety and tolerability of GV150526 in acute stroke (GLYB 2002). Cerebrovascular Diseases 1998;8(Supplement 4):20.
-
- Lees KR, Lavelle JF, Cunha L, Diener HC, Sanders EA, Tack P, et al. Glycine antagonist (GV150526) in acute stroke: a multicentre, double‐blind placebo‐controlled phase II trial. Cerebrovasc Dis 2001;11(1):20‐29. - PubMed
GLYB2003 {unpublished data only}
-
- Dyker AG, Lees KR. Safety and tolerability of GV150526 (a glycine site antagonist at the N‐ methyl‐D‐aspartate receptor) in patients with acute stroke. Stroke 1999;30(5):986‐992. - PubMed
IMAGES pilot {published data only}
-
- IMAGES Study Group, Bradford APJ, Muir KW, Lees KR. IMAGES pilot study of intravenous magnesium in acute stroke. Cerebrovascular Diseases 1998;8(Supplement 4):86.
LES 01 {unpublished data only}
-
- Depierre C. Clinical evaluation of eliprodil in the acute phase of stroke. Sanofi‐Synthelabo report 2000.
LES 02 {unpublished data only}
-
- Depierre C. Clinical evaluation of eliprodil in the acute phase of stroke. Sanofi‐Synthelabo report 2000.
Licostinel {published data only}
-
- Albers GW, Clark WM, Atkinson RP, Madden K, Data JL, Whitehouse MJ. Dose escalation study of the NMDA glycine‐site antagonist licostinel in acute ischemic stroke. Stroke 1999;30(3):508‐513. - PubMed
Lifarizine {published and unpublished data}
-
- Squire IB, Lees KR, Pryse‐Phillips W, Kertesz A, Bamford J. The effects of lifarizine in acute cerebral infarction: a pilot safety study. Cerebrovasc Dis 1996;6:156‐160.
Lub‐Int 13 {published data only}
-
- Diener HC, Cortens M, Ford G, Grotta J, Hacke W, Kaste M, et al. Lubeluzole in acute ischemic stroke treatment : A double‐blind study with an 8‐hour inclusion window comparing a 10‐mg daily dose of lubeluzole with placebo. Stroke 2000;31(11):2543‐2551. - PubMed
Lub‐Int 4 {published data only}
-
- Diener HC, Hacke W, Hennerici M, Radberg J, Hantson L, Keyser J. Lubeluzole in acute ischemic stroke. A double‐blind, placebo‐controlled phase II trial. Lubeluzole International Study Group. Stroke 1996;27:76‐81. - PubMed
Lub‐Int 5 {published and unpublished data}
-
- Diener HC. Multinational randomised controlled trial of lubeluzole in acute ischaemic stroke. European and Australian Lubeluzole Ischaemic Stroke Study Group. Cerebrovasc Dis 1998;8:172‐181. - PubMed
Lub‐Int 7 {published data only}
-
- Hacke W, Lees KR, Timmerhuis T, Haan J, Hantson L, Hennerici M, et al. Cardiovascular safety of lubeluzole (prosynap®) in patients with ischemic stroke. Cerebrovasc Dis 1998;8(5):247‐254. - PubMed
Lub‐Int 9 {published data only}
-
- Grotta J. Lubeluzole treatment of acute ischemic stroke. The US and Canadian Lubeluzole Ischemic Stroke Study Group. Stroke 1997;28:2338‐2346. - PubMed
Lub‐USA 6 {published data only}
Muir 1995a {published data only}
-
- Muir KW, Lees KR. A randomised, double‐blind, placebo‐controlled pilot trial of intravenous magnesium sulfate in acute stroke. Stroke 1995;26:183‐188. - PubMed
Muir 1998 {published data only}
-
- Muir KW, Lees KR. Dose optimization of intravenous magnesium sulfate after acute stroke. Stroke 1998;29:918‐923. - PubMed
Remacemide phase 2 {unpublished data only}
-
- Dyker AG, Lees KR. Remacemide hydrochloride: a double‐blind, placebo‐controlled, safety and tolerability study in patients with acute ischemic stroke. Stroke 1999;30(9):1796‐1801. - PubMed
Selfotel IIa {published data only}
-
- Grotta J, Clark W, Coull B, et al. Safety and tolerability of the glutamate antagonist CGS 19755 (selfotel) in patients with acute ischemic stroke: Results of a phase IIa randomized trial. Stroke 1995;26:602‐605. - PubMed
Selfotel IIb {published data only}
-
- Markabi S. Selfotel (CGS 19755): the preliminary clinical experience. In: Krieglstein J, Oberpichler‐Schwenk H editor(s). Pharmacology of Cerebral Ischemia. Stuttgart: Medpharm, 1994:635‐642.
Sipatrigine 137‐101 {published data only}
-
- Muir KW, Hamilton SJC, Lunnon MW, Hobbiger S, Lees KR. Safety and tolerability of 619C89 after acute stroke. Cerebrovasc Dis 1998;8:31‐37. - PubMed
Sipatrigine 137‐102 {unpublished data only}
-
- Hainsworth AH, Stefani A, Calabresi P, Smith TW, Leach MJ. Sipatrigine (BW619C89) is a neuroprotective agent and a sodium and calcium channel inhibitor. CNS Drug Reviews 2000;6(2):111‐134.
Sipatrigine 137‐104 {unpublished data only}
-
- Hainsworth AH, Stefani A, Calabresi P, Smith TW, Leach MJ. Sipatrigine (BW619C89) is a neuroprotective agent and a sodium and calcium channel inhibitor. CNS Drug Reviews 2000;6(2):111‐134.
Sipatrigine 137‐121 {published data only}
-
- Muir KW, Holzapfel L, Lees KR. Phase II clinical trial of sipatrigine (619C89) by continuous infusion in acute stroke. Cerebrovascular Diseases 2000;10(6):431‐436. - PubMed
Wester 1985 {published data only}
-
- Wester PO, Asplund K, Eriksson S, Hagg E, Lithner F, Strand T. Infusion of magnesium in patients with acute brain infarction. Acta Neurol Scand 1984;70(2):143. - PubMed
References to studies excluded from this review
Fosphenytoin phase 3 {published data only (unpublished sought but not used)}
-
- Multicenter Study to Evaluate the Safety and Efficacy of Intravenous Fosphenytoin in Patients with Acute Ischemic Stroke. Washington Stroke Center (http://www.strokecenter.org/trials/list/trialPage21.htm) 2001.
Galeas 1999 {published data only}
-
- Galeas T, Contos T, Exarhos P, Galea V, Valotasiou B. The role of magnesium (Mg) a natural calcium (Ca) antagonist in the treatment of acute ischaemic stroke (clinical study). Cerebrovascular Diseases 1999;9(Supplement 1):102.
Giroux 1994 {published data only}
-
- Giroux C, Garreau M, Morselli PL. Glutamate receptor antagonists in stroke patients. Neuropsychopharmacology 1994;10(Supplement 1):213S.
Lampl 2001 {published data only}
-
- Lampl Y, Gilad R, Geva D, Eshel Y, Sadeh M. Intravenous administration of magnesium sulfate in acute stroke: a randomized double‐blind study. Clin Neuropharmacol 2001;24(1):11‐15. - PubMed
Lub‐Int 2 & Lub‐Bel 4 {published data only}
-
- Keyser J, Velde V, Schellens RL, Hantson L, Tritsmans L, Gheuens J, et al. Safety and pharmacokinetics of the neuroprotective drug lubeluzole in patients with ischemic stroke. Clinical Therapeutics 1997;19(6):1340‐1351. - PubMed
Sipatrigine CEE 05 {unpublished data only}
-
- Sipatrigine. Unpublished.
ZK‐200775 {published data only (unpublished sought but not used)}
-
- Elting JW, Sulter GA, Kaste M, Lees KR, Diener HC, Hommel M, et al. AMPA antagonist ZK200775 in patients with acute ischemic stroke: possible glial cell toxicity detected by monitoring of S‐100B serum levels. Stroke 2002;33(12):2813‐2818. - PubMed
References to ongoing studies
ARTIST MRI {published and unpublished data}
-
- Yurko‐Mauro KA, Prince HC, Williams B, Tempel D, Warach S. ARTIST MRI: AMPA Receptor Antagonist Treatment in Ischemic Stroke Trial‐MRI. Proceedings of the 27th International Stroke Conference. 2002; Vol. Ongoing Clinical Trials:CTP349.
ARTIST+ {unpublished data only}
-
- Peterson L, Prince HC, Williams B, Tempel D, Grotta JC. ARTIST+: AMPA Receptor Antagonist Treatment in Ischemic Stroke Trial, YM872 + Alteplase. Proceedings of the 27th International Stroke Conference. American Stroke Association, 2002; Vol. Ongoing Clinical Trials:CTP350.
IMAGES {published and unpublished data}
Additional references
Aronowski 1996
-
- Aronowski J, Strong R, Grotta JC. Treatment of experimental focal ischemia in rats with lubeluzole. Neuropharmacology 1996;35(6):689‐693. - PubMed
Arrowsmith 1998
-
- Arrowsmith JE, Harrison MJ, Newman SP, Stygall J, Timberlake N, Pugsley WB. Neuroprotection of the brain during cardiopulmonary bypass: a randomized trial of remacemide during coronary artery bypass in 171 patients. Stroke 1998;29(11):2357‐2362. - PubMed
Bannan 1994
-
- Bannan PE, Graham DI, Lees KR, McCulloch J. Neuroprotective effect of remacemide hydrochloride in focal cerebral ischemia in the cat. Brain Res 1994;664:271‐275. - PubMed
Bialobok 1999
-
- Bialobok P, Cregan EF, Sydserff SG, Eisman MS, Miller JA, Cross AJ, et al. Efficacy of AR‐R15896AR in the rat monofilament model of transient middle cerebral artery occlusion. Journal of Stroke and Cerebrovascular Diseases 1999;8:388‐397. - PubMed
De Ryck 2000
-
- Ryck M, Verhoye M, Linden AM. Diffusion‐weighted MRI of infarct growth in a rat photochemical stroke model: effect of lubeluzole. Neuropharmacology 2000;39(4):691‐702. - PubMed
Dorman 1996
-
- Dorman PJ, Sandercock PA. Considerations in the design of clinical trials of neuroprotective therapy in acute stroke. Stroke 1996;27:1507‐1515. - PubMed
Gandolfo 2002
-
- Gandolfo C, Sandercock P, Conti M. Lubeluzole for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2002, Issue 4. [Art. No.: CD001924. DOI: 10.1002/14651858.CD001924] - PubMed
Grosset 1995
-
- Grosset DG, Muir KW, Lees KR. Systemic and cerebral hemodynamic responses to the non‐competitive NMDA antagonist CNS 1102. J Cardiovasc Pharmacol 1995;25:705‐709. - PubMed
Hainsworth 2000
-
- Hainsworth AH, Stefani A, Calabresi P, Smith TW, Leach MJ. Sipatrigine (BW619C89) is a neuroprotective agent and a sodium and calcium channel inhibitor. CNS Drug Reviews 2000;6(2):111‐134.
Horn 2001
-
- Horn J, Limburg M. Calcium antagonists for ischemic stroke: a systematic review. Stroke 2001;32(2):570‐576. - PubMed
Lekieffre 1997
-
- Lekieffre D, Benavides J, Scatton B, Nowicki JP. Neuroprotection afforded by a combination of eliprodil and a thrombolytic agent, rt‐PA, in a rat thromboembolic stroke model. Brain Res 1997;776(1‐2):88‐95. - PubMed
Mahoney 1965
-
- Mahoney FI, Barhel DW. Functional evaluation: the Barthel Index. Md State Med J 1965;14:61‐65. - PubMed
Maiese 1997
-
- Maiese K, TenBroeke M, Kue I. Neuroprotection of lubeluzole is mediated through the signal transduction pathways of nitric oxide. J Neurochem 1997;68(2):710‐714. - PubMed
McCulloch 1992
Meadows 1994
-
- Meadows M‐E, Fisher M, Minematsu K. Delayed treatment with a noncompetitive NMDA antagonist, CNS 1102, reduces infarct size in rats. Cerebrovasc Dis 1994;4:26‐31.
Minematsu 1993
-
- Minematsu K, Fisher M, Li L, Davis MA, Knapp AG, Cotter RE, et al. Effects of a novel NMDA antagonist on experimental stroke rapidly and quantitatively assessed by diffusion‐weighted MRI. Neurology 1993;43:397‐403. - PubMed
Morris 1999
-
- Morris GF, Bullock R, Marshall SB, Marmarou A, Maas A, Marshall LF. Failure of the competitive N‐methyl‐D‐aspartate antagonist Selfotel (CGS 19755) in the treatment of severe head injury: results of two phase III clinical trials. The Selfotel Investigators. J Neurosurg 1999;91:737‐743. - PubMed
Muir 1994
Muir 1995b
-
- Muir KW, Lees KR. Clinical experience with excitatory amino acid antagonist drugs. Stroke 1995;26(3):503‐513. - PubMed
Muir 1997
-
- Muir KW, Grosset DG, Lees KR. Effects of prolonged infusions of the NMDA antagonist aptiganel hydrochloride (CNS 1102) in normal volunteers. Clin Neuropharmacol 1997;20:311‐321. - PubMed
Muir 1999
-
- Muir KW, Grosset DG. Neuroprotection for acute stroke : making clinical trials work. Stroke 1999;30(1):180‐182. - PubMed
Muir 2002a
-
- Muir KW. Heterogeneity of stroke pathophysiology and neuroprotective clinical trial design. Stroke 2002;33(6):1545‐1550. - PubMed
NINDS 1995
-
- The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. The New England Journal of Medicine 1995;333(24):1581‐1587. - PubMed
NPS 1506
-
- Brady EM, Wells DS, Kierstead AE, Mueller AL, Newman MK, Sanguinetti EL, Marriott TB. Ascending dose safety and pharmacokinetic trial of NPS 1506, a novel NMDA receptor antagonist, in normal male volunteers. Neurology 1998;50(Supplement 1):A346.
Perez‐Pinzon 1995
-
- Perez‐Pinzon MA, Maier CM, Yoon EJ, Sun GH, Giffard RG, Steinberg GK. Correlation of CGS 19755 neuroprotection against in vitro excitotoxicity and focal cerebral ischemia. J Cereb Blood Flow Metab 1995;15(5):865‐876. - PubMed
Rankin 1957
-
- Rankin J. Cerebral vascular accidents in patients over the age of 60, II: prognosis. Scott Med J 1957;2:200‐215. - PubMed
Reggiani 2001
-
- Reggiani A, Pietra C, Arban R, Marzola P, Guerrini U, Ziviani L, et al. The neuroprotective activity of the glycine receptor antagonist GV150526: an in vivo study by magnetic resonance imaging. Eur J Pharmacol 2001;419(2‐3):147‐153. - PubMed
Scheller 1997
-
- Scheller DK, Ryck M, Kolb J, Szathmary S, Reempts J, Clincke G, et al. Lubeluzole blocks increases in extracellular glutamate and taurine in the peri‐infarct zone in rats. Eur J Pharmacol 1997;338(3):243‐251. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408. - PubMed
Smith 1997
-
- Smith SE, Hodges H, Sowinski P, Man CM, Leach MJ, Sinden JD, et al. Long‐term beneficial effects of BW619C89 on neurological deficit, cognitive deficit and brain damage after middle cerebral artery occlusion in the rat. Neuroscience 1997;77(4):1123‐1135. - PubMed
STAIR
-
- Stroke Therapy Academic Industry Roundtable (STAIR). Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 1999;30(12):2752‐2758. - PubMed
Takahashi 2002
Tirilazad
-
- Tirilazad International Steering Committee. Tirilazad mesylate in acute ischemic stroke: A systematic review. Stroke 2000;31(9):2257‐2265. - PubMed
Turski 1998
Van Swieten 1988
-
- Swieten JC, Koudstaal PJ, Visser MC, Schouten HJA, Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604‐607. - PubMed
Wahlgren 1999
-
- Wahlgren NG, Ranasinha KW, Rosolacci T, Franke CL, Erven PM, Ashwood T, et al. Clomethiazole acute stroke study (CLASS) : results of a randomized, controlled trial of clomethiazole versus placebo in 1360 acute stroke patients. Stroke 1999;30(1):21‐28. - PubMed
Yenari 1998
-
- Yenari MA, Bell TE, Kotake AN, Powell M, Steinberg GK. Dose escalation safety and tolerance study of the competitive NMDA antagonist selfotel (CGS 19755) in neurosurgery patients. Clin Neuropharmacol 1998;21(1):28‐34. - PubMed
YM872
-
- Napoliello MJ, Voss RW. Clinical experience with YM872, a novel AMPA receptor antagonist for acute ischemic stroke. Stroke 1999;30(1):264.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

