G-CSF and GM-CSF for treating or preventing neonatal infections
- PMID: 12917944
- PMCID: PMC7016516
- DOI: 10.1002/14651858.CD003066
G-CSF and GM-CSF for treating or preventing neonatal infections
Abstract
Background: The colony stimulating factors (CSFs), granulocyte-macrophage colony stimulating factor (GM-CSF) and granulocyte colony stimulating factor (G-CSF), are naturally occurring cytokines that stimulate the production and antibacterial function of neutrophils and monocytes. Two strategies have been adopted for exploring whether CSFs can provide clinical benefit for preterm infants. The first has investigated their use as a treatment to improve outcome in established systemic infection, especially when complicated by a low neutrophil count. The alternative strategy has been to use CSFs prophylactically, to prevent sepsis prospectively through stimulation of neutrophil production and bactericidal function.
Objectives: To determine the efficacy and safety of the haemopoietic colony stimulating factors (G-CSF or GM-CSF) in newborn infants, when used for:a) treatment of suspected or proven systemic infection to reduce mortality, orb) prophylaxis, to prevent systemic infection in infants at high risk of nosocomial infection. To determine, in subgroup analysis, the influence of pre-existing or high risk of neutropenia on the outcome of therapy.
Search strategy: PubMed, EMBASE, MEDLINE and the Cochrane Central Register of Controlled Trials (The Cochrane Library, Issue 2, 2003) were searched in April 2003 using the keywords: G-CSF, GM-CSF, infant newborn, with and without the limit Clinical Trial. In addition, reference lists of identified RCTs, meta-analyses and personal files were searched.
Selection criteria: The criteria used to select studies for inclusion were:
Design: RCT.
Subjects: Newborn infants in intensive care.
Interventions: G-CSF or GM-CSF given as treatment in conjunction with antibiotics for suspected or microbiologically proven systemic infection. G-CSF or GM-CSF given as prophylaxis with the aim of reducing the incidence of systemic infection.
Outcomes: Treatment studies reporting all cause mortality. Prophylaxis studies reporting subsequent incidence of sepsis and / or mortality.
Data collection and analysis: Relative risks (RR) and risk differences (RD) with 95% confidence intervals (CI) using the fixed effect model are reported. Number needed to treat (NNT) was calculated for the outcomes that showed a statistically significant reduction in RR.
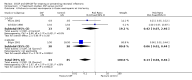
Main results: Seven treatment studies of 257 infants with suspected systemic bacterial infection and three prophylaxis studies comprising 359 neonates are analysed. Treatment studies: There is no evidence that the addition of G-CSF or GM-CSF to antibiotic therapy in preterm infants with suspected systemic infection reduces immediate all cause mortality. No significant survival advantage was seen at 14 days from the start of therapy [typical RR 0.71 (95% CI 0.38,1.33); typical RD -0.05 (95% CI -0.14, 0.04)]. However all seven of the treatment studies were small, the largest recruiting only 60 infants. The subgroup analysis of 97 infants from three treatment studies who, in addition to systemic infection, had clinically significant neutropenia (< 1.7 x 10(9)/l) at trial entry, does show a significant reduction in mortality by day 14 [RR 0.34 (95% CI 0.12, 0.92); RD -0.18 (95% CI -0.33, -0.03); NNT 6 (95% CI 3-33)]. Prophylaxis studies have not demonstrated a significant reduction in mortality in neonates receiving GM-CSF [RR 0.59 (95% CI 0.24,1.44); RD -0.03 (95% CI -0.08,0.02)]. The identification of sepsis as the primary outcome of prophylaxis studies has been hampered by inadequately stringent definitions of systemic infection. However, data from one study suggest that prophylactic GM-CSF may provide protection against infection when given to preterm infants who are neutropenic or at high risk of developing postnatal neutropenia.
Reviewer's conclusions: There is currently insufficient evidence to support the introduction of either G-CSF or GM-CSF into neonatal practice, either as treatment of established systemic infection to reduce resulting mortality, or as prophylaxis to prevent systemic infection in high risk neonates. No toxicity of CSF use was reported in any systemic infection to reduce resulting mortality, or as prophylaxis to prevent systemic infection in high risk neonates. No toxicity of CSF use was reported in any study included in this review. The limited data suggesting that CSF treatment may reduce mortality when systemic infection is accompanied by severe neutropenia should be investigated further in adequately powered trials which recruit sufficient infants infected with organisms associated with a significant mortality risk.
Conflict of interest statement
The authors were the investigators responsible for one prophylaxis study included in this review (Carr 1999).
The authors are currently investigating the effect of prophylactic GM‐CSF in neonates at high risk for neutropenia in a large multicentre randomised controlled trial in the UK (PROGRAMS).
Figures










References
References to studies included in this review
Ahmad 2002 {published data only}
-
- Ahmad A, Laborada G, Bussel J, Nesin M. Comparison of recombinant G‐CSF, recombinant human GM‐CSF and placebo for treatment of septic preterm infants. Pediatric Infectious Disease Journal 2002;21:1061‐5. - PubMed
Bedford‐Russell 2001 {published and unpublished data}
-
- Bedford‐Russell AR, Emmerson AJB, Wilkinson N, Chant T, Sweet DG, Halliday HL, Holland B, Davies EG. A trial of recombinant human granulocyte colony stimulating factor for the treatment of very low birthweight infants with presumed sepsis and neutropenia. Archives of Disease in Childhood. Fetal and Neonatal Edition 2001;84:F172‐6. - PMC - PubMed
Bilgin 2001 {published data only}
-
- Bilgin K, Yaramis A, Haspolat K, Tas A, Gunbey S, Derman O. A randomized trial of granulocyte‐macrophage colony‐stimulating factor in neonates with sepsis and neutropenia. Pediatrics 2001;107:36‐41. - PubMed
Cairo 1995 {published data only}
-
- Cairo MS, Christensen RD, Sender LS, Ellis R, Rosenthal J, Ven C, Worcester C, Agosti JM. Results of a phase I/II trial of recombinant human granulocyte‐macrophage colony‐stimulating factor in very low birthweight neonates: significant induction of circulatory neutrophils, monocytes, platelets, and bone marrow neutrophils. Blood 1995;86:2509‐15. - PubMed
Cairo 1999 {published data only}
-
- Cairo MS, Agosti J, Ellis R, Laver JJ, Puppala B, DeLemos R, Givner L, Nesin M, Wheeler G, Seth T, Ven C, Fanaroff A. A randomised double‐blind placebo‐controlled trial of prophylactic recombinant human GM‐CSF to reduce nosocomial infection in very low birthweight neonates. Journal of Pediatrics 1999;134:64‐70. - PubMed
Carr 1999 {published and unpublished data}
-
- Carr R, Modi N, Doré CJ, El‐Rifai, Lindo D. A randomised controlled trial of prophylactic GM‐CSF in human newborns less than 32 weeks gestation. Pediatrics 1999;103:796‐802. - PubMed
Drossou‐Agakidou '98 {published data only}
-
- Drossou‐Agakidou V, Kanakoudi‐Tsakalidou F, Taparkou A, Tzimouli V, Tsandali H, Kremenopoulos G. Administration of recombinant human granulocyte‐colony stimulating factor to septic neonates induces neutrophilia and enhances the neutrophil respiratory burst and beta2 integrin expression Results of a randomized controlled trial. Neonatology 1998;157:583‐8. - PubMed
Gillan 1994 {published data only}
-
- Gillan ER, Christensen RD, Suen Y, et al. A randomized, placebo‐controlled trial of recombinant human granulocyte colony‐stimulating factor administration in newborn infants with presumed sepsis: significant induction of peripheral and bone marrow neutrophilia. Blood 1994;84:1427‐33. - PubMed
-
- Rosenthal J, Healey T, Ellis R, Gillan E, Cairo M. A two year follow‐up of neonates with presumed sepsis treated with recombinant human G‐CSF during the first week of life. Journal of Pediatrics 1996;128:135‐7. - PubMed
Miura 2001 {published data only}
-
- Miura E, Procianoy RS, Bittar C, Miura CS, Miura MS, Mello C, Christensen RD. A randomized double‐masked, placebo controlled trial of recombinant granulocyte colony‐stimulating factor administration to preterm infants with the clinical diagnosis of early‐onset sepsis. Pediatrics 2001;107:30‐5. - PubMed
Schibler 1998 {published data only}
-
- Schibler KR, Osborne KA, Leung LY, Le TV, Baker SI, Thompson DD. A randomized placebo‐controlled trial of granulocyte colony‐stimulating factor administration to newborn infants with neutropenia and clinical signs of early‐onset sepsis. Pediatrics 1998;102:6‐13. - PubMed
References to studies awaiting assessment
Kucukoduk 2002 {published data only}
-
- Kucukoduk S, Sezer T, Yildiran A, Albayrak D. Randomised, double‐blind, placebo controlled trial of early administration of recombinant human G‐CSF to non‐neutropenic preterm newborns between 33 and 36 weeks, with presumed sepsis. Scandinavian Journal of Infectious Diseases 2002;34:893‐7. - PubMed
La Gamma 2000 {published data only}
-
- Gamma E, Welch W, Jeffs R. A multicentre, double‐blind, randomised, placebo‐controlled safety and efficacy study of filgrastim (rHu‐G‐CSF) in the treament of late‐onset neonatal sepsis. Pediatric Research 2000;47:409A.
References to ongoing studies
PROGRAMS {unpublished data only}
-
- Modi N, Carr R, Brocklehurst P. PROGRAMS.
Additional references
Bernstein 2001
-
- Bernstein HM, Pollock BH, Calhoun DA, Christensen RD. Administration of recombinant G‐CSF to neonates with septicaemia: a meta‐analysis. Pediatrics 2001;138:917‐20. - PubMed
Carr 1997
Carr 2000a
-
- Carr R. Neutrophil production and function in newborn infants. British Journal of Haematology 2000;110:18‐28. - PubMed
Carr 2000b
Carr 2003
-
- Carr R, Modi N. Haemopoietic growth factors for neonates: assessing risks and benefits. Acta Paediatrica 2003:In Press. - PubMed
Christensen 1989
-
- Christensen RD. Neutrophil kinetics in the fetus and neonate. Am J Pediatr Hematol Oncol 1989;11:215‐223. - PubMed
Cooke 1997
-
- Cooke RW, Nycyk JA, Okuonghuae H, et al. Low dose vancomycin prophylaxis reduces coagulase‐negative staphylococcal bacteraemia in very low birthweight infants. J Hosp Infect 1997;37:297‐303. - PubMed
Damman 1998
-
- Damman O, Leviton A. Infection remote from the brain, neonatal white matter damage, and cerebral palsy in the preterm infant. Semin Pediatr Neurol 1998;5:190‐201. - PubMed
Engle 1984
-
- Engle WD, Rosenfeld CR. Neutropenia in high risk neonates. J Pediatr 1984;105:982‐986. - PubMed
Gessler 1995
-
- Gessler P, Luders R, Konig S, et al. Neonatal neutropenia in low birthweight premature infants. Am J Perinatol 1995;12:34‐38. - PubMed
Hill 1987
-
- Hill HR. Biochemical, structural, and functional abnormalities of polymorphonuclear leukocytes in the neonate. Pediatr Res 1987;22:375‐382. - PubMed
Kocherlakota 1998
-
- Kocherlakota P, Gamma EF. Preliminary report: rhG‐CSF may reduce the incidence of neonatal sepsis in prolonged preeclampsia‐associated neutropenia. Pediatrics 1998;102:1107‐1111. - PubMed
Koening 1989
-
- Koenig JH, Christensen RD. Incidence, neutrophil kinetics and natural history of neonatal neutropenia associated with maternal hypertension. N Eng J Med 1989;321:557‐62. - PubMed
Lejeune 1999
-
- Lejeune M, Cantineiux B, Harag S, et al. Defective functional activity and accelerated apoptosis in neutrophils from children with cancer are differentially corrected by granulocyte and granulocyte‐macrophage colony stimulating factors in vitro. Br J Haematol 1999;106:756‐761. - PubMed
Manroe 1979
-
- Manroe BL, Weinberg AG, Rosenfeld CR, Browne R. The neonatal blood count in health and disease. i. Reference values for neutrophilic cells. J Pediatr 1979;95:89‐98. - PubMed
Modi 2000
Roberts 1991
-
- Roberts RL, Szelc CM, Scates SM, Boyd MT, Soderstrom KM, Davis MW, Glaspy JA. Neutropenia in an extremely premature infant treated with recombinant human granulocyte colony‐stimulating factor. Am J Dis Child 1991;145:808‐812. - PubMed
Rodwell 1993
-
- Rodwell RL, Taylor KMCD, Tudehope DI, Gray PH. Hematologic scoring system in early diagnosis of sepsis in neutropenic newborns. Pediatr Inf Dis J 1993;12:372‐376. - PubMed
Stoll 1996
-
- Stoll BJ, Gordon T, Korones SB, et al. Late‐onset sepsis in very low birthweight neonates: A report from the National Institute of Child Health and Human Development neonatal research network. J Pediatr 1996;129:63‐71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

