Oestrogen supplementation, mainly diethylstilbestrol, for preventing miscarriages and other adverse pregnancy outcomes
- PMID: 12918007
- PMCID: PMC9039959
- DOI: 10.1002/14651858.CD004353
Oestrogen supplementation, mainly diethylstilbestrol, for preventing miscarriages and other adverse pregnancy outcomes
Abstract
Background: Laboratory evidence in the 1940s demonstrated a positive role of placental hormones in the continuation of pregnancy. It was suggested that diethylstilbestrol was the oestrogen of choice for prevention of miscarriages. Observational studies were carried out with apparently positive results, on which clinical practice was based. This led to a worldwide usage of diethylstilbestrol despite controlled studies with contrary findings.
Objectives: To determine the effects of antenatal administration of oestrogens, mainly diethylstilbestrol, on high risk and unselected pregnancy as regards miscarriages and other outcomes.
Search strategy: We searched the Pregnancy and Childbirth Group Specialised Register of controlled trials in November 2002.
Selection criteria: Randomised and quasi-randomised trials were included.
Data collection and analysis: Both reviewers extracted data from the studies identified that met the selection criteria, and the data were analysed using the RevMan software.
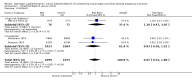
Main results: Miscarriage, preterm labour, low birthweight and stillbirth or neonatal death were not positively influenced by the intervention (diethylstilbestrol) as compared to the control group. Diethylstilbestrol in utero exposure led to increased rate of miscarriage and preterm birth. There was also an increase in the numbers of babies weighing less than 2500 grams. The maternal outcome in terms of pre-eclampsia was not influenced. Exposed female offsprings have a non-significant trend towards more cancer of the genital tract and cancer other than of the genital tract. Primary infertility, adenosis of the vagina/cervix in female offsprings, and testicular abnormality in male offsprings were significantly higher in those exposed to diethylstilbestrol before birth.
Reviewer's conclusions: There was no benefit with the use of diethylstilbestrol in preventing miscarriages. Both short and long-term adverse outcomes in exposed offsprings were demonstration of the harm that this intervention caused women and their offspring during its usage.
Conflict of interest statement
None known.
Figures















References
References to studies included in this review
Bender 1988 {unpublished data only}
-
- Bender S. The effect of diethylstilboestrol on recurrent miscarriage. Personal communication February 1988.
Crowder 1950 {published data only}
-
- Crowder RE, Bills ES, Broadbent JS. The management of threatened abortion. A study of 100 cases. American Journal of Obstetrics and Gynecology 1950 Oct;60(4):896‐9. - PubMed
Dieckmann 1953 {published data only}
-
- Bibbo M, Al‐Naqeeb M, Baccarini I, Gill W, Newton M, Sleeper KM, et al. Follow‐up study of male and female offspring of DES‐treated mothers. A preliminary report. Journal of Reproductive Medicine 1975;15(1):29‐32. - PubMed
-
- Bibbo M, Gill W, Azizi F, Blough R, Fang VS, Rosenfield RL, et al. Follow‐up study of male and female offspring of DES‐exposed mothers. Obstetrics and Gynecology 1977;49(1):1‐8. - PubMed
-
- Bibbo M, Haemszel WM, Wied GL, Hubby M, Herbst AL. A twenty year follow‐up study of women exposed to diethylstilbestrol during pregnancy. New England Journal of Medicine 1978;298(14):763‐7. - PubMed
-
- Brackbill Y, Berendes H. Dangers of diethylstilbestrol: review of a 1953 paper. Lancet 1978;2:520. - PubMed
-
- Dieckmann WJ, Davis ME, Rynkiewcz SM, Pottinger RE. Does the administration of diethylstilbestrol during pregnancy have therapeutic value?. American Journal of Obstetrics and Gynecology 1953;66(5):1062‐81. - PubMed
Ferguson 1953 {published data only}
-
- Ferguson JH. Effect of stilbestrol on pregnancy compared to the effect of a placebo. American Journal of Obstetrics and Gynecology 1953;65(3):592‐601. - PubMed
MRC and follow up {published data only}
-
- Medical Research Council. The use of hormones in the management of pregnancy in diabetics. Lancet 1955;2:833‐6. - PubMed
-
- Vessey MP, Fairweather DVI, Norman‐Smith B, Buckley J. A randomized double‐blind controlled trial of the value of stilboestrol therapy in pregnancy: long term follow‐up of mothers and their offspring. British Journal of Obstetrics and Gynaecology 1983;90:1007‐17. - PubMed
Robinson 1952 {published data only}
-
- Robinson D, Shettles LB. The use of diethylstilboestrol in threatened abortion. American Journal of Obstetrics and Gynecology 1952;63(6):1330‐3. - PubMed
Swyer 1954 {published data only}
-
- Swyer GIM, Law RG. An evaluation of the prophylactic ante‐natal use of stilboestrol. Preliminary report. Proceedings of the society for endocrinology. Journal of Endocrinology 1954;10:36‐7.
References to studies excluded from this review
Baird 1996 {published data only}
-
- Baird DD, Wilcox AJ, Herbst AL. Self‐reported allergy, infection and autoimmune diseases among men and women exposed in utero to diethylstilboestrol. Journal of Clinical Epidemiology 1996;49(2):263‐6. - PubMed
Berle 1977 {published data only}
-
- Berle P, Behnke K. The treatment of threatened abortion [Uber Behandlungserfolge der drohenden Fehlgeburt]. Geburtshilfe und Frauenheilkunde 1977;37:139‐42. - PubMed
Berle 1980 {published data only}
-
- Berle P, Budenz M, Michaelis J. Is hormonal therapy still justified in imminent abortions? [Besitzt die Hormontherapie bei der Behandlung des Abortus immens eine Berechtigung?]. Zeitschrift fur Geburtshilfe und Perinatologie 1980;184:353‐8. - PubMed
Kaufman 2000 {published data only}
-
- Kaufman RH, Adam E, Hatch EE, Noller K, Herbst AL, Palmer JR, et al. Continued follow up of pregnancy outcomes in diethylstilbestrol‐exposed offspring. Obstetrics and Gynecology 2000;96(4):483‐9. - PubMed
Smith 1949 {published data only}
-
- Smith OW, S Smith G. The influence of diethylstilbestrol on the progress and outcome of pregnancy as based on a comparison of treated with untreated primigravidas. American Journal of Obstetrics and Gynecology 1949;58:994‐1006. - PubMed
Additional references
Bibbo 1975
-
- Bibbo M, Al‐Naqeeb M, Baccarini I, Gill W, Newton M, Sleeper KM, et al. Follow‐up study of male and female offspring of DES‐treated mothers. A preliminary report. Journal of Reproductive Medicine 1975;15:29‐32. - PubMed
Bibbo 1977
-
- Bibbo M, Gill WB, Azizi P, Blough R, Fang VS, Rosenfield RL, et al. Follow‐up study of male and female offspring of DES‐exposed mothers. Obstetrics and Gynecology 1977;49:1‐8. - PubMed
Everett 1997
FDA 1971
-
- FDA Drug Bulletin. Diethylstilboestrol contraindicated in pregnancy. US Dept of Health, Education and Welfare 1971.
Herbst 1971
-
- Herbst AL, Ulfelder H, Poskanzer DC. Association of maternal stilboestrol therapy with tumour appearance in young women. New England Journal of Medicine 1971;284:878‐81. - PubMed
Herbst 1975
-
- Herbst AL, Poskanzer DC, Robboy SJ, Friedlander L, Scully RE, et al. Prenatal exposure to stilboestrol. A prospective comparison of exposed female offspring with unexposed controls. New England Journal of Medicine 1975;292:334‐9. - PubMed
Pencharz 1940
-
- Pencharz RI. Effect of estrogens and androgens alone and in combination with chorionic gonadotrophin on ovary of hypophysectomized rat. Science 1940;91:554. - PubMed
Smith 1941
-
- Smith OW, Smith GV, Schiller S. Estrogen and progestin metabolism in pregnancy: spontaneous and induced labor. Journal of Clinical Endocrinology 1941;1:461‐9.
Smith 1944
-
- Smith OW, Smith GVS. Pituitary stimulating property of stilbestrol as compared with that of estrone. Proceedings of the Society for Experimental Biology and Medicine 1944;57:198‐200.
Smith 1948
-
- Smith OW. Diethylstilboestrol in the prevention and treatment of complications of pregnancy. American Journal of Obstetrics and Gynecology 1948;56:821‐34. - PubMed
References to other published versions of this review
Beral 1995
-
- Beral V, Chalmers I. Diethylstilboestrol (DES) in pregnancy. [ revised 21 April 1994] In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

