Contribution of eIF-4E inhibition to the expression and activity of heparanase in human colon adenocarcinoma cell line: LS-174T
- PMID: 12918105
- PMCID: PMC4611528
- DOI: 10.3748/wjg.v9.i8.1707
Contribution of eIF-4E inhibition to the expression and activity of heparanase in human colon adenocarcinoma cell line: LS-174T
Abstract
Aim: Heparanase degrades heparan sulfate proteoglycans (HSPGs) and is a critical mediator of tumor metastasis and angiogenesis. Recently, it has been cloned as a single gene family and found to be a potential target for antimetastasis drugs. However, the molecular basis for the regulation of heparanase expression is still not quite clear. The aim of this study was to determine whether the expression of eukaryotic initiation factor 4E (eIF-4E) correlated with the heparanase level in tumor cells and to explore the correlation between heparanase expression and metastatic potential of LS-174T cells.
Methods: A 20-mer antisense s-oligodeoxynucleotide (asODN) targeted against the translation start site of eIF-4E mRNA was introduced into LS-174T cells by lipid-mediated DNA-transfection. eIF-4E protein and mRNA levels were detected by Western blot analysis and RT-PCR, respectively. Heparanase activity was defined as the ability to degrade high molecular weight (40-100 kDa) radiolabeled HS (heparan sulfate) substrate into low molecular weight (5-15 kDa) HS fragments that could be differentiated by gel filtration chromatography. The invasive potential of tumor cell in vitro was observed by using a Matrigel invasion assay system.
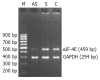
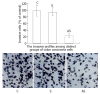
Results: The 20-mer asODN against eIF-4E specifically and significantly inhibited eIF-4E expression at both transcriptional and translational levels. As a result, the expression and activity of heparanase were effectively retarded and the decreased activity of heparanase resulted in the decreased invasive potential of LS-174T.
Conclusion: eIF-4E is involved in the regulation of heparanase production in colon adenocarcinoma cell line LS-174T, and its critical function makes it a particularly interesting target for heparanase regulation. This targeting strategy in antisense chemistry may have practical applications in experimental or clinical anti-metastatic gene therapy of human colorectal carcinoma.
Figures



References
-
- Poste G, Fidler IJ. The pathogenesis of cancer metastasis. Nature. 1980;283:139–146. - PubMed
-
- Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. - PubMed
-
- Duffy MJ. The role of proteolytic enzymes in cancer invasion and metastasis. Clin Exp Metastasis. 1992;10:145–155. - PubMed
-
- Toyoshima M, Nakajima M. Human heparanase. Purification, characterization, cloning, and expression. J Biol Chem. 1999;274:24153–24160. - PubMed
-
- Iozzo RV, Murdoch AD. Proteoglycans of the extracellular environment: clues from the gene and protein side offer novel perspectives in molecular diversity and function. FASEB J. 1996;10:598–614. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

