Pharmacodynamic characterization of the interaction between the glycoprotein IIb/IIIa inhibitor YM337 and unfractionated heparin and aspirin in humans
- PMID: 12919181
- PMCID: PMC1884347
- DOI: 10.1046/j.0306-5251.2003.01873.x
Pharmacodynamic characterization of the interaction between the glycoprotein IIb/IIIa inhibitor YM337 and unfractionated heparin and aspirin in humans
Abstract
Aims: To investigate the pharmacodynamic interaction of unfractionated heparin (UFH) and acetylic salicylic acid (ASA) on YM337, a monoclonal humanized antibody of the platelet GPIIb/IIIa receptor.
Methods: In a randomized, placebo-controlled study three treatment groups each with six healthy volunteers received the following medication: group 1, ASA (3 days) + UFH + YM337 (placebo); group 2, ASA (placebo) + UFH (placebo) + YM337; group 3, ASA + UFH + YM337. Assessments were made over 24 h and included bleeding time (BT), ADP (20 microm)- and collagen (5 microg ml-1)-induced platelet aggregation and PAC1 and CD62 expression measured by flow cytometry.
Results: In group 3 BT was prolonged to 35 [median, 16-45 min (1,3 quartile)] after UFH administration, increasing to 45 [median, 42-45 min (1,3 quartile)] after YM infusion (6 h). BT remained elevated to 26 [median, 14-45 min (1,3 quartile)] at 24 h, while groups 1 and 2 returned to normal values. Collagen-induced aggregation was 73% [median, 70-80% (1,3 quartile)] under YM337 alone, 79% [median, 72-80% (1,3 quartile)] under ASA + UFH and reduced only in group 3 to 24% [median, 18-29% (1,3 quartile)]. In both groups receiving active YM337, PAC1 expression showed a reduction to < 20% after 6 h of infusion. CD62 expression was not significantly affected by any treatment.
Conclusion: UFH and YM337 have strong synergistic effects on BT, while coadministration of ASA strongly augments inhibitory effects of YM337 on collagen-induced platelet aggregation.
Figures

 Acetylic salicylic acid (ASA) + unfractionated heparin (UFH),
Acetylic salicylic acid (ASA) + unfractionated heparin (UFH),  YM337, □ ASA + UFH + YM337. *P < 0.02 from corresponding value of treatment with YM337 alone.
YM337, □ ASA + UFH + YM337. *P < 0.02 from corresponding value of treatment with YM337 alone.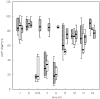
 acetylic salicylic acid (ASA) + unfractionated heparin (UFH),
acetylic salicylic acid (ASA) + unfractionated heparin (UFH),  YM337, □ ASA + UFH + YM337, P < 0.02 from corresponding value of treatment with YM337 alone.
YM337, □ ASA + UFH + YM337, P < 0.02 from corresponding value of treatment with YM337 alone.
 acetylic salicylic acid (ASA) + unfractionated heparin (UFH),
acetylic salicylic acid (ASA) + unfractionated heparin (UFH),  YM337, □ ASA + UFH + YM337. *P < 0.02 from corresponding value of treatment with YM337 alone.
YM337, □ ASA + UFH + YM337. *P < 0.02 from corresponding value of treatment with YM337 alone.Similar articles
-
Pharmacodynamic characterization of the interaction between abciximab or tirofiban with unfractionated or a low molecular weight heparin in healthy subjects.Br J Clin Pharmacol. 2001 Sep;52(3):297-305. doi: 10.1046/j.0306-5251.2001.01446.x. Br J Clin Pharmacol. 2001. PMID: 11560562 Free PMC article. Clinical Trial.
-
Pharmacokinetics and pharmacodynamic effects of a new antibody glycoprotein IIb/IIIa inhibitor (YM337) in healthy subjects.Circulation. 1999 Sep 14;100(11):1175-81. doi: 10.1161/01.cir.100.11.1175. Circulation. 1999. PMID: 10484537 Clinical Trial.
-
Antiplatelet and antithrombotic effects of YM337, the Fab fragment of a humanized anti-GPIIb/IIIa monoclonal antibody in monkeys.Thromb Haemost. 1996 Apr;75(4):679-84. Thromb Haemost. 1996. PMID: 8743199
-
Pharmacodynamic and clinical trials of glycoprotein IIb/IIIa inhibitors and potential relationship of results to dosing.Z Kardiol. 2003 Mar;92(3):213-8. doi: 10.1007/s00392-003-0895-6. Z Kardiol. 2003. PMID: 12658467 Review.
-
Counteracting the effects of anticoagulants and antiplatelet agents during neurosurgical emergencies.Neurosurgery. 2005 Nov;57(5):823-31; discussion 823-31. doi: 10.1227/01.neu.0000179915.74429.b2. Neurosurgery. 2005. PMID: 16284551 Review.
Cited by
-
Giving monoclonal antibodies to healthy volunteers in phase 1 trials: is it safe?Br J Clin Pharmacol. 2013 Aug;76(2):164-72. doi: 10.1111/bcp.12096. Br J Clin Pharmacol. 2013. PMID: 23438102 Free PMC article. Review.
-
Platelet integrin αIIbβ3: signal transduction, regulation, and its therapeutic targeting.J Hematol Oncol. 2019 Mar 7;12(1):26. doi: 10.1186/s13045-019-0709-6. J Hematol Oncol. 2019. PMID: 30845955 Free PMC article. Review.
-
Population pharmacokinetics-pharmacodynamics of alemtuzumab (Campath) in patients with chronic lymphocytic leukaemia and its link to treatment response.Br J Clin Pharmacol. 2007 Sep;64(3):278-91. doi: 10.1111/j.1365-2125.2007.02914.x. Epub 2007 May 16. Br J Clin Pharmacol. 2007. PMID: 17506867 Free PMC article. Clinical Trial.
-
Anti-platelet therapy with clopidogrel prevents endothelial dysfunction and vascular remodeling in aortas from hypertensive rats.PLoS One. 2014 Mar 17;9(3):e91890. doi: 10.1371/journal.pone.0091890. eCollection 2014. PLoS One. 2014. PMID: 24638017 Free PMC article.
References
-
- Kong DF, Califf RM, Miller DP, et al. Clinical outcomes of therapeutic agents that block the platelet glycoprotein IIb/IIIa integrin in ischemic heart disease. Circulation. 1998;98:2829–2835. - PubMed
-
- Brener SJ, Barr LA, Burchenal JE, et al. Randomized, placebo-controlled trial of platelet glycoprotein IIb/IIIa blockade with primary angioplasty for acute myocardial infarction. ReoPro and Primary PTCA Organization and Randomized Trial (RAPPORT) Investigators. Circulation. 1998;98:734–741. - PubMed
-
- The EPILOG Investigators. Platelet glycoprotein IIb/IIIa receptor blockade and low-dose heparin during percutaneous coronary revascularization. N Engl J Med. 1997;336:1689–1696. - PubMed
-
- Reverter JC, Beguin S, Kessels H, Kumar R, Hemker HC, Coller BS. Inhibition of platelet mediated, tissue factor-induced thrombin generation by the mouse/human chimeric 7E3 antibody. Potential implications for the effect of c7E3 Fab treatment on acute thrombosis and ‘clinical restenosis’. J Clin Invest. 1996;98:863–874. - PMC - PubMed
-
- Harder S, Kirchmaier CM, Krzywanek J, Westrup D, Bae JW, Breddin HK. Pharmacokinetics and pharmacodynamic effects of a new antibody glycoprotein IIb/IIIa inhibitor (YM 337) in healthy subjects. Circulation. 1999;100:1175–1182. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

