Tacrolimus dose requirement in renal transplant recipients is significantly higher when used in combination with corticosteroids
- PMID: 12919182
- PMCID: PMC1884345
- DOI: 10.1046/j.0306-5251.2003.01882.x
Tacrolimus dose requirement in renal transplant recipients is significantly higher when used in combination with corticosteroids
Abstract
Aims: To evaluate the effect of corticosteroids on tacrolimus pharmacokinetics.
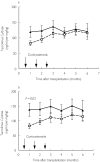
Methods: In a randomized trial, kidney transplant recipients were treated with tacrolimus and mycophenolate mofetil with either daclizumab (n = 31) or 3 months of prednisone (n = 34). Tacrolimus dose-adjusted predose concentrations (C0) at month 1-6 were compared between both groups and within the corticosteroid group before and after prednisone withdrawal.
Results: At month 1 the tacrolimus dose-adjusted C0 in the corticosteroid group was 83 +/- 8 vs 119 +/- 17 ng ml-1 mg-1 kg-1 in the daclizumab group. The tacrolimus dose-adjusted C0 within the corticosteroid group at month 1 and 2 was 42% and 29% lower compared with month 4 (P < 0.001).
Conclusions: A higher tacrolimus dose is required to reach target concentrations when used in combination with corticosteroids.
Figures
References
-
- Winkler M, Christians U. A risk-benefit assessment of tacrolimus in transplantation. Drug Saf. 1995;12:348–357. - PubMed
-
- Oellerich M, Armstrong VW, Schutz E, Shaw LM. Therapeutic drug monitoring of cyclosporine and tacrolimus. Update on Lake Louise Consensus Conference on cyclosporin and tacrolimus. Clin Biochem. 1998;31:309–316. - PubMed
-
- Krahenbuhl S, Menafoglio A, Giostra E, Gallino A. Serious interaction between mibefradil and tacrolimus. Transplantation. 1998;66:1113–1115. - PubMed
-
- Chenhsu RY, Loong CC, Chou MH, Lin MF, Yang WC. Renal allograft dysfunction associated with rifampin–tacrolimus interaction. Ann Pharmacother. 2000;34:27–31. - PubMed
-
- Moreno M, Latorre A, Manzanares C, et al. Clinical management of tacrolimus drug interactions in renal transplant patients. Transplant Proc. 1999;31:2252–2253. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

