Structural maturation of rubella virus in the Golgi complex
- PMID: 12919732
- PMCID: PMC7119121
- DOI: 10.1016/s0042-6822(03)00384-2
Structural maturation of rubella virus in the Golgi complex
Abstract
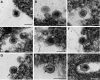
Rubella virus is a small enveloped virus that assembles in association with Golgi membranes. Freeze-substitution electron microscopy of rubella virus-infected cells revealed a previously unrecognized virion polymorphism inside the Golgi stacks: homogeneously dense particles without a defined core coexisting with less dense, mature virions that contained assembled cores. The homogeneous particles appear to be a precursor form during the virion morphogenesis process as the forms with mature morphology were the only ones detected inside secretory vesicles and on the exterior of cells. In mature virions potential remnants of C protein membrane insertion were visualized as dense strips connecting the envelope with the internal core. In infected cells Golgi stacks were frequently seen close to cytopathic vacuoles, structures identified as the sites for viral RNA replication, along with the rough endoplasmic reticulum and mitochondria. These associations could facilitate the transfer of viral genomes from the cytopathic vacuoles to the areas of rubella assembly in Golgi membranes.
Figures



Similar articles
-
Three-dimensional structure of Rubella virus factories.Virology. 2010 Sep 30;405(2):579-91. doi: 10.1016/j.virol.2010.06.043. Epub 2010 Jul 23. Virology. 2010. PMID: 20655079 Free PMC article.
-
Polymorphism and structural maturation of bunyamwera virus in Golgi and post-Golgi compartments.J Virol. 2003 Jan;77(2):1368-81. doi: 10.1128/jvi.77.2.1368-1381.2003. J Virol. 2003. PMID: 12502853 Free PMC article.
-
Novel replication complex architecture in rubella replicon-transfected cells.Cell Microbiol. 2007 Apr;9(4):875-90. doi: 10.1111/j.1462-5822.2006.00837.x. Epub 2006 Nov 3. Cell Microbiol. 2007. PMID: 17087733 Free PMC article.
-
Role of rubella virus glycoprotein domains in assembly of virus-like particles.J Virol. 1999 May;73(5):3524-33. doi: 10.1128/JVI.73.5.3524-3533.1999. J Virol. 1999. PMID: 10196241 Free PMC article.
-
Golgi apparatus analyzed by cryo-electron microscopy.Histochem Cell Biol. 2013 Oct;140(4):369-81. doi: 10.1007/s00418-013-1136-3. Epub 2013 Aug 18. Histochem Cell Biol. 2013. PMID: 23954988 Free PMC article. Review.
Cited by
-
Accommodation of large cargo within Golgi cisternae.Histochem Cell Biol. 2013 Sep;140(3):261-9. doi: 10.1007/s00418-013-1120-y. Epub 2013 Jul 3. Histochem Cell Biol. 2013. PMID: 23821163 Free PMC article. Review.
-
Chondroitin sulfate N-acetylgalactosaminyltransferase-2 contributes to the replication of infectious bursal disease virus via interaction with the capsid protein VP2.Viruses. 2015 Mar 23;7(3):1474-91. doi: 10.3390/v7031474. Viruses. 2015. PMID: 25807054 Free PMC article.
-
Key Golgi factors for structural and functional maturation of bunyamwera virus.J Virol. 2005 Sep;79(17):10852-63. doi: 10.1128/JVI.79.17.10852-10863.2005. J Virol. 2005. PMID: 16103138 Free PMC article.
-
Antiviral activity of hemolymph of Podalia against rubella virus.Cytotechnology. 2017 Feb;69(1):31-37. doi: 10.1007/s10616-016-0035-6. Epub 2016 Nov 28. Cytotechnology. 2017. PMID: 27896559 Free PMC article.
-
Molecular and Structural Insights into the Life Cycle of Rubella Virus.J Virol. 2021 Apr 26;95(10):e02349-20. doi: 10.1128/JVI.02349-20. Epub 2021 Feb 24. J Virol. 2021. PMID: 33627388 Free PMC article. Review.
References
-
- Bardeletti G, Tektoff J, Gautheron D. Rubella virus maturation and production in two host cell systems. Intervirology. 1979;11:97–103. - PubMed
-
- Baron M.D, Ebel T, Suomalainen M. Intracellular transport of rubella virus structural proteins expressed from cloned cDNA. J. Gen. Virol. 1992;73:1073–1086. - PubMed
-
- Chantler J, Wolinsky J.S, Tingle A. Rubella virus. In: Knipe D.M, Howley P.M, editors. Fields Virology. Lippincott Williams & Wilkins; Philadelphia, PA: 2001. pp. 963–990.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

