Cyclooxygenase-1 and cyclooxygenase-2 in the mouse ductus arteriosus: individual activity and functional coupling with nitric oxide synthase
- PMID: 12922938
- PMCID: PMC1573985
- DOI: 10.1038/sj.bjp.0705391
Cyclooxygenase-1 and cyclooxygenase-2 in the mouse ductus arteriosus: individual activity and functional coupling with nitric oxide synthase
Abstract
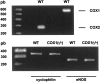
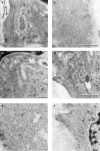
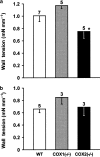
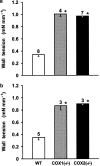
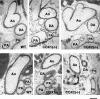
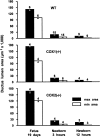
1. Prenatal patency of the ductus arteriosus is maintained by prostaglandin (PG) E(2), conceivably in concert with nitric oxide (NO). Local PGE(2) formation is sustained by cyclooxygenase-1 (COX1) and cyclooxygenase-2 (COX2), a possible exception being the mouse in which COX1, or both COXs, are reportedly absent. Here, we have examined the occurrence of functional COX isoforms in the near-term mouse ductus and the possibility of COX deletion causing NO upregulation. 2. COX1 and COX2 were detected in smooth muscle cells by immunogold electronmicroscopy, both being located primarily in the perinuclear region. Cytosolic and microsomal PGE synthases (cPGES and mPGES) were also found, but they occurred diffusely across the cytosol. COX1 and, far more frequently, COX2 were colocalised with mPGES, while neither COX appeared to be colocalized with cPGES. 3. The isolated ductus from wild-type and COX1-/- mice contracted promptly to indomethacin (2.8 micro M). Conversely, the contraction of COX2-/- ductus to the same inhibitor started only after a delay and was slower. 4. N(G)-nitro-L-arginine methyl ester (L-NAME, 100 micro M) weakly contracted the isolated wild-type ductus. Its effect, however, increased three- to four-fold after deleting either COX, hence equalling that of indomethacin. 5. In vivo, the ductus was patent in all mice foetuses, whether wild-type or COX-deleted. Likewise, no genotype-related difference was noted in its postnatal closure. 6. We conclude that the mouse ductus has a complete system for PGE(2) synthesis comprising both COX1 and COX2. The two enzymes respond differently to indomethacin but, nevertheless, deletion of either one results in NO upregulation. PGE(2) and NO can function synergistically in keeping the ductus patent.
Figures



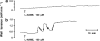
References
-
- BOUAYAD A., KAJINO H., WALEH N., FOURON J.-C., ANDELFINGER G., VARMA D.R., SKOLL A., VAZQUEZ A., GOBEIL F., JR, CLYMAN R.I., CHEMTOB S. Characterisation of PGE receptors in fetal and newborn lamb ductus arteriosus. Am. J. Physiol. 2001;280:H2342–H2349. - PubMed
-
- CHALLIS J.R.G., DILLEY S.R., ROBINSON J.S., THORBURN G.D. Prostaglandins in the circulation of the fetal lamb. Prostaglandins. 1976;11:1041–1052. - PubMed
-
- CHOMCZYNSKY P., SACCHI N. Single-step method of RNA isolation by acid guanidinium thyocyanate–phenol–chloroform extraction. Anal. Biochem. 1987;162:156–159. - PubMed
-
- CLYMAN R.I. Ductus arteriosus: current theories of prenatal and postnatal regulation. Semin. Perinatol. 1987;11:64–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

