Development of the heart: (3) formation of the ventricular outflow tracts, arterial valves, and intrapericardial arterial trunks
- PMID: 12923046
- PMCID: PMC1767864
- DOI: 10.1136/heart.89.9.1110
Development of the heart: (3) formation of the ventricular outflow tracts, arterial valves, and intrapericardial arterial trunks
Figures


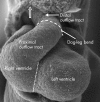
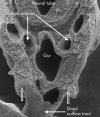

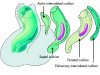



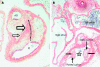
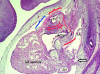
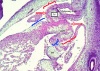

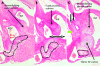
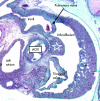
References
-
- Le Douarin NM. The neural crest. Cambridge: Cambridge University Press, 1982.
-
- Kirby ML, Bockman DE. Neural crest and normal development: a new perspective. Anat Rec 1984;209:1–6. ▸ It was the pioneering work of Le Dourain that first showed the importance of the neural crest in development, but Kirby and her colleagues then extended this work to show the significance of migrations of cells from extracardiac sources to formation of the cardiac outflow tracts and arch arteries. - PubMed
-
- Ya J, van den Hoff MJB, de Boer PAJ, et al. The normal development of the outflow tract in the rat. Circ Res 1998;82:464–72. ▸ The first work to question the conventional division of the outflow tract into “truncus” and “conus”, showing how, during development, there was a retreat of the myocardium initially clothing the entirety of the developing outflow tract. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
