Heritable activity: a prion that propagates by covalent autoactivation
- PMID: 12923060
- PMCID: PMC196450
- DOI: 10.1101/gad.1115803
Heritable activity: a prion that propagates by covalent autoactivation
Abstract
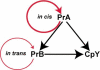
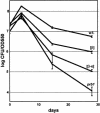
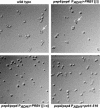
Known prions (infectious proteins) are self-propagating amyloids or conformationally altered proteins, but in theory an enzyme necessary for its own activation could also be a prion (or a gene composed of protein). We show that yeast protease B is such a prion, called [beta].[beta] is infectious, reversibly curable, and its de novo generation is induced by overexpression of the pro-protease. Present in normal cells but masked by the functionally redundant protease A, [beta] is advantageous during starvation and necessary for sporulation. We propose that other enzymes whose active, modified, form is necessary for their maturation might also be prions.
Figures

Similar articles
-
The reconstitution of mammalian prion infectivity de novo.FEBS J. 2007 Feb;274(3):576-87. doi: 10.1111/j.1742-4658.2007.05630.x. FEBS J. 2007. PMID: 17288547 Review.
-
[Yeast prions, mammalian amyloidoses, and the problem of proteomic networks].Genetika. 2006 Nov;42(11):1558-70. Genetika. 2006. PMID: 17163073 Review. Russian.
-
Prion genetics: new rules for a new kind of gene.Annu Rev Genet. 2004;38:681-707. doi: 10.1146/annurev.genet.38.072902.092200. Annu Rev Genet. 2004. PMID: 15355224 Review.
-
Protein-only transmission of three yeast prion strains.Nature. 2004 Mar 18;428(6980):319-23. doi: 10.1038/nature02391. Nature. 2004. PMID: 15029195
-
Amyloidogenic domains, prions and structural inheritance: rudiments of early life or recent acquisition?Curr Opin Chem Biol. 2004 Dec;8(6):665-71. doi: 10.1016/j.cbpa.2004.09.002. Curr Opin Chem Biol. 2004. PMID: 15556413 Review.
Cited by
-
PrionHome: a database of prions and other sequences relevant to prion phenomena.PLoS One. 2012;7(2):e31785. doi: 10.1371/journal.pone.0031785. Epub 2012 Feb 20. PLoS One. 2012. PMID: 22363733 Free PMC article.
-
Anti-Prion Systems in Saccharomyces cerevisiae Turn an Avalanche of Prions into a Flurry.Viruses. 2022 Sep 1;14(9):1945. doi: 10.3390/v14091945. Viruses. 2022. PMID: 36146752 Free PMC article. Review.
-
[NSI (+)]: a novel non-Mendelian nonsense suppressor determinant in Saccharomyces cerevisiae.Curr Genet. 2010 Oct;56(5):467-78. doi: 10.1007/s00294-010-0314-2. Epub 2010 Jul 29. Curr Genet. 2010. PMID: 20668856
-
Amyloid Fragmentation and Disaggregation in Yeast and Animals.Biomolecules. 2021 Dec 15;11(12):1884. doi: 10.3390/biom11121884. Biomolecules. 2021. PMID: 34944528 Free PMC article. Review.
-
Prions are affected by evolution at two levels.Cell Mol Life Sci. 2016 Mar;73(6):1131-44. doi: 10.1007/s00018-015-2109-6. Epub 2015 Dec 28. Cell Mol Life Sci. 2016. PMID: 26713322 Free PMC article. Review.
References
-
- Bueler H., Fischer, M., Lang, Y., Bluethmann, H., Lipp, H.P., DeArmond, S.J., Prusiner, S.B., Aguet, M., and Weissmann, C. 1992. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature 356: 577–582. - PubMed
-
- Caughey B. 2000. Transmissible spongiform encephalopathies, amyloidoses and yeast prions: Common threads? Nat. Med. 6: 751–754. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
