Coordinate regulation of the Escherichia coli formate dehydrogenase fdnGHI and fdhF genes in response to nitrate, nitrite, and formate: roles for NarL and NarP
- PMID: 12923080
- PMCID: PMC180993
- DOI: 10.1128/JB.185.17.5076-5085.2003
Coordinate regulation of the Escherichia coli formate dehydrogenase fdnGHI and fdhF genes in response to nitrate, nitrite, and formate: roles for NarL and NarP
Abstract
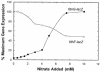
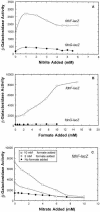
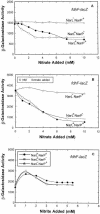
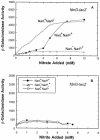
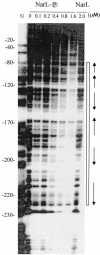
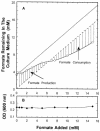
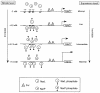
Escherichia coli possesses three distinct formate dehydrogenase enzymes encoded by the fdnGHI, fdhF, and fdoGHI operons. To examine how two of the formate dehyrogenase operons (fdnGHI and fdhF) are expressed anaerobically in the presence of low, intermediate, and high levels of nitrate, nitrite, and formate, chemostat culture techniques were employed with fdnG-lacZ and fdhF-lacZ reporter fusions. Complementary patterns of gene expression were seen. Optimal fdhF-lacZ expression occurred only at low to intermediate levels of nitrate, while high nitrate levels caused up to 10-fold inhibition of gene expression. In contrast, fdnG-lacZ expression was induced 25-fold in the presence of intermediate to high nitrate concentrations. Consistent with prior reports, NarL was able to induce fdnG-lacZ expression. However, NarP could not induce expression; rather, it functioned as an antagonist of fdnG-lacZ expression under low-nitrate conditions (i.e., it was a negative regulator). Nitrite, a reported signal for the Nar sensory system, was unable to stimulate or suppress expression of either formate dehydrogenase operon via NarL and NarP. The different gene expression profiles of the alternative formate dehydrogenase operons suggest that the two enzymes have complementary physiological roles under environmental conditions when nitrate and formate levels are changing. Revised regulatory schemes for NarL- and NarP-dependent nitrate control are presented for each operon.
Figures

Similar articles
-
The nrfA and nirB nitrite reductase operons in Escherichia coli are expressed differently in response to nitrate than to nitrite.J Bacteriol. 2000 Oct;182(20):5813-22. doi: 10.1128/JB.182.20.5813-5822.2000. J Bacteriol. 2000. PMID: 11004182 Free PMC article.
-
Synthetic lac operator substitutions for studying the nitrate- and nitrite-responsive NarX-NarL and NarQ-NarP two-component regulatory systems of Escherichia coli K-12.J Bacteriol. 2003 Apr;185(7):2104-11. doi: 10.1128/JB.185.7.2104-2111.2003. J Bacteriol. 2003. PMID: 12644479 Free PMC article.
-
Nitrate and nitrite regulation of the Fnr-dependent aeg-46.5 promoter of Escherichia coli K-12 is mediated by competition between homologous response regulators (NarL and NarP) for a common DNA-binding site.J Mol Biol. 1995 Aug 4;251(1):15-29. doi: 10.1006/jmbi.1995.0412. J Mol Biol. 1995. PMID: 7643383
-
Nitrate regulation of anaerobic respiratory gene expression in Escherichia coli.Mol Microbiol. 1993 Aug;9(3):425-34. doi: 10.1111/j.1365-2958.1993.tb01704.x. Mol Microbiol. 1993. PMID: 8412692 Review.
-
Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors.Biochim Biophys Acta. 1997 Jul 4;1320(3):217-34. doi: 10.1016/s0005-2728(97)00034-0. Biochim Biophys Acta. 1997. PMID: 9230919 Review.
Cited by
-
Association between Intestinal Colonization and Extraintestinal Infection with Carbapenem-Resistant Klebsiella pneumoniae in Children.Microbiol Spectr. 2023 Mar 14;11(2):e0408822. doi: 10.1128/spectrum.04088-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36916927 Free PMC article.
-
Molybdenum- and tungsten-containing formate dehydrogenases and formylmethanofuran dehydrogenases: Structure, mechanism, and cofactor insertion.Protein Sci. 2019 Jan;28(1):111-122. doi: 10.1002/pro.3498. Epub 2018 Oct 31. Protein Sci. 2019. PMID: 30120799 Free PMC article.
-
Molybdenum Enzymes and How They Support Virulence in Pathogenic Bacteria.Front Microbiol. 2020 Dec 11;11:615860. doi: 10.3389/fmicb.2020.615860. eCollection 2020. Front Microbiol. 2020. PMID: 33362753 Free PMC article. Review.
-
Engineering transcription factors with novel DNA-binding specificity using comparative genomics.Nucleic Acids Res. 2009 May;37(8):2493-503. doi: 10.1093/nar/gkp079. Epub 2009 Mar 5. Nucleic Acids Res. 2009. PMID: 19264798 Free PMC article.
-
Identification of a novel nutrient-sensing histidine kinase/response regulator network in Escherichia coli.J Bacteriol. 2014 Jun;196(11):2023-9. doi: 10.1128/JB.01554-14. Epub 2014 Mar 21. J Bacteriol. 2014. PMID: 24659770 Free PMC article.
References
-
- Birkmann, A., and A. Bock. 1989. Characterization of a cis regulatory DNA element necessary for formate induction of the formate dehydrogenase gene (fdhF) of Escherichia coli. Mol. Microbiol. 3:187-195. - PubMed
-
- Birkmann, A., R. G. Sawers, and A. Bock. 1987. Involvement of the ntrA gene product in the anaerobic metabolism of Escherichia coli. Mol. Gen. Genet. 210:535-542. - PubMed
-
- Blattner, F. R., G. Plunkett 3rd, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. - PubMed
-
- Bock, A., and G. Sawers. 1996. Fermentation, p. 262-282. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. American Society for Microbiology, Washington, D.C.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

