Purification and characterization of the flagellar basal body of Rhodobacter sphaeroides
- PMID: 12923105
- PMCID: PMC181020
- DOI: 10.1128/JB.185.17.5295-5300.2003
Purification and characterization of the flagellar basal body of Rhodobacter sphaeroides
Abstract
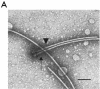
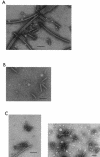
Flagellar hook-basal body (HBB) complexes were purified from Rhodobacter sphaeroides. The HBB was more acid labile but more heat stable than that of Salmonella species, and protein identification revealed that HBB components were expressed only from one of the two sets of flagellar gene clusters on the R. sphaeroides genome, under the heterotrophic growth conditions tested here.
Figures


Similar articles
-
Purification of Fla2 Flagella of Rhodobacter sphaeroides.Methods Mol Biol. 2017;1593:273-283. doi: 10.1007/978-1-4939-6927-2_22. Methods Mol Biol. 2017. PMID: 28389962
-
Isolation and ultrastructural study of the flagellar basal body complex from Rhodobacter sphaeroides WS8 (wild type) and a polyhook mutant PG.Biochem Biophys Res Commun. 1997 Sep 29;238(3):733-7. doi: 10.1006/bbrc.1997.7359. Biochem Biophys Res Commun. 1997. PMID: 9325158
-
Structural Characterization of the Fla2 Flagellum of Rhodobacter sphaeroides.J Bacteriol. 2015 Sep;197(17):2859-66. doi: 10.1128/JB.00170-15. Epub 2015 Jun 29. J Bacteriol. 2015. PMID: 26124240 Free PMC article.
-
Living in a Foster Home: The Single Subpolar Flagellum Fla1 of Rhodobacter sphaeroides.Biomolecules. 2020 May 16;10(5):774. doi: 10.3390/biom10050774. Biomolecules. 2020. PMID: 32429424 Free PMC article. Review.
-
Rhodobacter sphaeroides: complexity in chemotactic signalling.Trends Microbiol. 2008 Jun;16(6):251-60. doi: 10.1016/j.tim.2008.02.006. Epub 2008 Apr 25. Trends Microbiol. 2008. PMID: 18440816 Review.
Cited by
-
Chemotactic control of the two flagellar systems of Rhodobacter sphaeroides is mediated by different sets of CheY and FliM proteins.J Bacteriol. 2007 Nov;189(22):8397-401. doi: 10.1128/JB.00730-07. Epub 2007 Sep 21. J Bacteriol. 2007. PMID: 17890312 Free PMC article.
-
Genome sequence of Rhodobacter sphaeroides Strain WS8N.J Bacteriol. 2011 Aug;193(15):4027-8. doi: 10.1128/JB.05257-11. Epub 2011 May 27. J Bacteriol. 2011. PMID: 21622735 Free PMC article.
-
A distant homologue of the FlgT protein interacts with MotB and FliL and is essential for flagellar rotation in Rhodobacter sphaeroides.J Bacteriol. 2013 Dec;195(23):5285-96. doi: 10.1128/JB.00760-13. Epub 2013 Sep 20. J Bacteriol. 2013. PMID: 24056105 Free PMC article.
-
Characterization of lateral flagella of Selenomonas ruminantium.Appl Environ Microbiol. 2011 Apr;77(8):2799-802. doi: 10.1128/AEM.00286-11. Epub 2011 Feb 18. Appl Environ Microbiol. 2011. PMID: 21335384 Free PMC article.
-
Motor-driven bacterial flagella and buckling instabilities.Eur Phys J E Soft Matter. 2012 Feb;35(2):15. doi: 10.1140/epje/i2012-12015-0. Epub 2012 Feb 29. Eur Phys J E Soft Matter. 2012. PMID: 22395533
References
-
- Goodfellow, I. G. P.1996. Ph.D. thesis. University of Nottingham, Nottingham, United Kingdom.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

