Bacillus subtilis diacylglycerol kinase (DgkA) enhances efficient sporulation
- PMID: 12923107
- PMCID: PMC180973
- DOI: 10.1128/JB.185.17.5306-5309.2003
Bacillus subtilis diacylglycerol kinase (DgkA) enhances efficient sporulation
Abstract
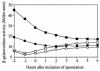
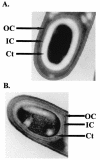
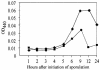
The sn-1,2-diacylglycerol kinase homologue gene, dgkA, is a sporulation gene indispensable for the maintenance of spore stability and viability in Bacillus subtilis. After 6 h of growth in resuspension medium, the endospore morphology of the dgkA mutant by standard phase-contrast microscopy was normal; however, after 9 h, the endospores appeared mostly dark by phase-contrast microscopy, suggesting a defect in the spores. Moreover, electron microscopic studies revealed an abnormal cortex structure in mutant endospores 6 h after the onset of sporulation, an indication of cortex degeneration. In addition, a significant decrease in the dipicolinic acid content of mutant spores was observed. We also found that dgkA is expressed mainly during the vegetative phase. It seems likely that either the DgkA produced during growth prepares the cell for an essential step in sporulation or the enzyme persists into sporulation and performs an essential function.
Figures

References
-
- Dowhan, W. 1997. Molecular basis for membrane phospholipid diversity: why are there so many lipids? Annu. Rev. Biochem. 66:199-232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

