Cellular interactions in the rat somatosensory thalamocortical system during normal and epileptic 5-9 Hz oscillations
- PMID: 12923213
- PMCID: PMC2343451
- DOI: 10.1113/jphysiol.2003.046573
Cellular interactions in the rat somatosensory thalamocortical system during normal and epileptic 5-9 Hz oscillations
Abstract
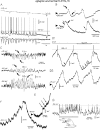
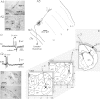
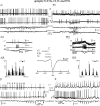
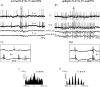
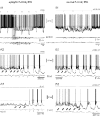
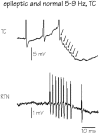
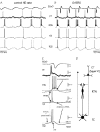
In Genetic Absence Epilepsy Rats from Strasbourg (GAERS), generalized spike-and-wave (SW) discharges (5-9 SW s(-1)) develop during quiet immobile wakefulness from a natural, medium-voltage, 5-9 Hz rhythm. This study examines the spatio-temporal dynamics of cellular interactions in the somatosensory thalamocortical system underlying the generation of normal and epileptic 5-9 Hz oscillations. Paired single-unit and multi-unit recordings between the principal elements of this circuit and intracellular recordings of thalamic, relay and reticular, neurones were conducted in neuroleptanalgesied GAERS and control, non-epileptic, rats. The identity of the recorded neurones was established following juxtacellular or intracellular marking. At least six major findings have emerged from this study. (1) In GAERS, generalized spike-and-wave discharges were correlated with synchronous rhythmic firings in related thalamic relay and reticular neurones. (2) Usually, corticothalamic discharges phase-led related relay and reticular firings. (3) A depolarizing wave emerging from a barrage of EPSPs was the cause of both relay and reticular discharges. (4) In some relay cells, which had a relatively high membrane input resistance, the depolarizing wave had the shape of a ramp, which could trigger a low-threshold Ca2+ spike. (5) In reticular cells, the EPSP barrage could further trigger voltage-dependent depolarizations. (6) The epilepsy-related thalamic, relay and reticular, intracellular activities were similar to the normal-related thalamic activities. Overall, these findings strongly suggest that, during absence seizures, corticothalamic neurones play a primary role in the synchronized excitation of thalamic relay and reticular neurones. The present study further suggests that absence-related spike-and-wave discharges correspond to hypersynchronous wake-related physiological oscillations.
Figures





References
-
- Andrade R. Blockade of neurotransmitter-activated K+ conductance by QX-314 in the rat hippocampus. Eur J Pharmacol. 1991;19:259–262. - PubMed
-
- Avoli M, Gloor P. Interaction of cortex and thalamus in spike and wave discharges of feline generalized penicillin epilepsy. Exp Neurol. 1982;76:196–217. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

