Protein kinase C and A sites on troponin I regulate myofilament Ca2+ sensitivity and ATPase activity in the mouse myocardium
- PMID: 12923217
- PMCID: PMC2343448
- DOI: 10.1113/jphysiol.2003.045260
Protein kinase C and A sites on troponin I regulate myofilament Ca2+ sensitivity and ATPase activity in the mouse myocardium
Abstract
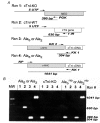
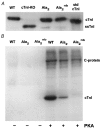
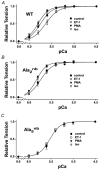
Cardiac troponin I (cTnI) is a phosphoprotein subunit of the troponin-tropomyosin complex that is thought to inhibit cardiac muscle contraction during diastole. To investigate the contributions of cTnI phosphorylation to cardiac regulation, transgenic mice were created with the phosphorylation sites of cTnI mutated to alanine. Activation of protein kinase C (PKC) by perfusion of hearts with phorbol-12-myristate-13-acetate (PMA) or endothelin-1 (ET-1) inhibited the maximum ATPase rate by up to 25 % and increased the Ca2+ sensitivity of ATPase activity and of isometric tension by up to 0.15 pCa units. PKC activation no longer altered cTnI phosphorylation, depressed ATPase rates or enhanced myofilament Ca2+ sensitivity in transgenic mice expressing cTnI that could not be phosphorylated on serines43/45 and threonine144 (PKC sites). Modest changes in myosin regulatory light chain phosphorylation occurred in all mouse lines, but increases in myofilament Ca2+ sensitivity required the presence of phosphorylatable cTnI. For comparison, the beta-adrenergic agonist isoproterenol caused a 38 % increase in maximum ATPase rate and a 0.12 pCa unit decrease in myofilament Ca2+ sensitivity. These beta-adrenergic effects were absent in transgenic mice expressing cTnI that could not be phosphorylated on serines23/24 (protein kinase A, PKA, sites). Overall, the results indicate that PKC and PKA exert opposing effects on actomyosin function by phosphorylating cTnI on distinct sites. A primary role of PKC phosphorylation of cTnI may be to reduce the requirements of the contractile apparatus for both Ca2+ and ATP, thereby promoting efficient ATP utilisation during contraction.
Figures



References
-
- Adelstein RS, Klee CB. Purification and characterization of smooth muscle myosin light chain kinase. J Biol Chem. 1981;256:7501–7509. - PubMed
-
- Andersen GG, Qvigstad E, Schiander I, Aass H, Osnes JB, Skomedal T. Alpha(1)AR-induced positive inotropic response in heart is dependent on myosin light chain phosphorylation. Am J Physiol. 2002;283:H1471–1480. - PubMed
-
- Burkart EM, Sumandea MP, Kobayashi T, Nili M, Martin AF, Homsher E, Solaro RJ. Phosphorylation or glutamic acid substitution at protein kinase C sites on cardiac troponin I differentially depress myofilament tension and shortening velocity. J Biol Chem. 2003;278:11265–11272. - PubMed
-
- Cohen MV, Baines CP, Downey JM. Ischemic preconditioning: from adenosine receptor of KATP channel. Annu Rev Physiol. 2000;62:79–109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

