An endonuclease activity similar to Xenopus PMR1 catalyzes the degradation of normal and nonsense-containing human beta-globin mRNA in erythroid cells
- PMID: 12923263
- PMCID: PMC1370479
- DOI: 10.1261/rna.5720303
An endonuclease activity similar to Xenopus PMR1 catalyzes the degradation of normal and nonsense-containing human beta-globin mRNA in erythroid cells
Abstract
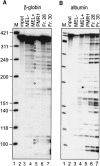
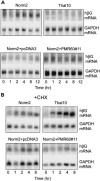
beta-globin mRNA bearing a nonsense codon is degraded in the cytoplasm of erythroid cells by endonuclease cleavage, preferentially at UG dinucleotides. An endonuclease activity in polysomes of MEL cells cleaved beta-globin and albumin mRNA in vitro at many of the same sites as PMR1, an mRNA endonuclease purified from Xenopus liver. Stable transfection of MEL cells expressing normal human beta-globin mRNA with a plasmid vector expressing the catalytically active form of PMR1 reduced the half-life of beta-globin mRNA from 12 to 1-2 h without altering GAPDH mRNA decay. The reduced stability of beta-globin mRNA in these cells was accompanied by an increase in the production of mRNA decay products corresponding to those seen in the degradation of nonsense-containing beta-globin mRNA. Therefore, beta-globin mRNA is cleaved in vivo by an endonuclease with properties similar to PMR1. Inhibiting translation with cycloheximide stabilized nonsense-containing beta-globin mRNA, resulting in a fivefold increase in its steady-state level. Taken together, our results indicate that the surveillance of nonsense-containing beta-globin mRNA in erythroid cells is a cytoplasmic process that functions on translating mRNA, and endonucleolytic cleavage constitutes one step in the process of beta-globin mRNA decay.
Figures




References
-
- Antoniou, M. 1991. Induction of erythroid-specific expression in murine erythroleukemia (MEL) cell line. In Methods in molecular biology. Gene transfer and expression protocols (ed. E.J. Murray), pp. 421–434. Humana Press, Clifton, NJ. - PubMed
-
- Antoniou, M. and Grosveld, F. 1990. β-globin dominant control region interacts differently with distal and proximal promoter elements. Genes & Dev. 4: 1007–1013. - PubMed
-
- Binder, R., Horowitz, J.A., Basilion, J.P., Koeller, D.M., Klausner, R.D., and Harford, J.B. 1994. Evidence that the pathway of transferrin receptor mRNA degradation involves an endonucleolytic cleavage within the 3′ UTR and does not involve poly(A) tail shortening. EMBO J. 13: 1969–1980. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
