Regulation of BMP-induced transcription in cultured human bone marrow stromal cells
- PMID: 12925605
- PMCID: PMC1351076
- DOI: 10.2106/00004623-200300003-00005
Regulation of BMP-induced transcription in cultured human bone marrow stromal cells
Abstract
Background: Adherent bone marrow stromal cells are inducible osteoprogenitors, giving rise to cells expressing osteoblast markers including alkaline phosphatase, osteopontin, osteocalcin, and bone sialoprotein. However, the potency of inducers varies in a species-specific manner. Glucocorticoids such as dexamethasone induce alkaline phosphatase activity in both human and rat mesenchymal stem cells, while mouse bone marrow stromal cells are refractory to dexamethasone-induced alkaline phosphatase activity. In contrast, BMP induces alkaline phosphatase activity in both mouse and rat bone marrow stromal cells, while BMP effects on human bone marrow stromal cells are poorly characterized.
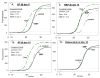
Methods: Bone marrow samples were isolated from patients undergoing hip replacement. Mononuclear marrow cells were cultured and grown to confluence without or with 10 (-7) M dexamethasone. Cells from each isolate were passaged into medium containing 100 micro g/mL ascorbate phosphate and treated with dexamethasone, 100 ng/mL BMP, or no inducer. At day 6, alkaline phosphatase activity was assayed, and RNA was prepared for mRNA analyses by real-time polymerase chain reaction.
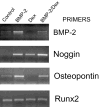
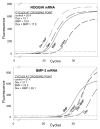
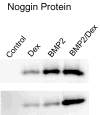
Results: Bone marrow stromal cells from twenty-four of twenty-six patients showed no significant osteogenic response to BMP-2, 4, or 7 as determined by alkaline phosphatase induction. However, BMPs induced elevated levels of other genes associated with osteogenesis such as bone sialoprotein and osteopontin as well as BMP-2 and noggin. If primary cultures of human bone marrow stromal cells were pretreated with dexamethasone, BMP-2 treatment of first-passage cells induced alkaline phosphatase in approximately half of the isolates, and significantly greater induction was seen in cells from males. Dexamethasone treatment, like BMP treatment, also increased expression of the BMP-binding protein noggin.
Conclusions: Most human femur bone marrow stromal cell samples appear incapable of expressing elevated alkaline phosphatase levels in response to BMPs. Since BMP treatment induced expression of several other BMP-regulated genes, the defect in alkaline phosphatase induction is presumably not due to impaired BMP signaling. We hypothesize that the mechanism by which BMPs modulate alkaline phosphatase expression is indirect, involving a BMP-regulated transcription factor for alkaline phosphatase expression that is controlled differently in humans and rodents.
Figures

Similar articles
-
Different effects of BMP-2 on marrow stromal cells from human and rat bone.Cells Tissues Organs. 2004;176(1-3):109-19. doi: 10.1159/000075032. Cells Tissues Organs. 2004. PMID: 14745240 Free PMC article.
-
BMP responsiveness in human mesenchymal stem cells.Connect Tissue Res. 2003;44 Suppl 1:305-11. Connect Tissue Res. 2003. PMID: 12952214
-
Induction of rapid osteoblast differentiation in rat bone marrow stromal cell cultures by dexamethasone and BMP-2.Dev Biol. 1994 Jan;161(1):218-28. doi: 10.1006/dbio.1994.1022. Dev Biol. 1994. PMID: 8293874
-
Targeting bone morphogenetic protein antagonists: in vitro and in vivo evidence of their role in bone metabolism.Expert Opin Ther Targets. 2009 Jan;13(1):123-37. doi: 10.1517/14728220802637725. Expert Opin Ther Targets. 2009. PMID: 19063711 Review.
-
The function of adipocytes in the bone marrow stroma: an update.Bone. 1996 Nov;19(5):421-8. doi: 10.1016/s8756-3282(96)00258-x. Bone. 1996. PMID: 8922639 Review.
Cited by
-
The Effects of Stably Tethered BMP-2 on MC3T3-E1 Preosteoblasts Encapsulated in a PEG Hydrogel.Biomacromolecules. 2021 Mar 8;22(3):1065-1079. doi: 10.1021/acs.biomac.0c01085. Epub 2021 Feb 8. Biomacromolecules. 2021. PMID: 33555180 Free PMC article.
-
The transcription factor osterix (SP7) regulates BMP6-induced human osteoblast differentiation.J Cell Physiol. 2012 Jun;227(6):2677-85. doi: 10.1002/jcp.23010. J Cell Physiol. 2012. PMID: 21898406 Free PMC article.
-
Considerations of growth factor and material use in bone tissue engineering using biodegradable scaffolds in vitro and in vivo.Sci Rep. 2024 Oct 28;14(1):25832. doi: 10.1038/s41598-024-75198-3. Sci Rep. 2024. PMID: 39468149 Free PMC article.
-
Saving Implants BMP-2 Application in Revision Total Hip Surgery.Int J Biomed Sci. 2006 Jun;2(2):187-95. Int J Biomed Sci. 2006. PMID: 23674982 Free PMC article.
-
Improving Osteogenesis Activity on BMP-2-Immobilized PCL Fibers Modified by the γ-Ray Irradiation Technique.Biomed Res Int. 2015;2015:302820. doi: 10.1155/2015/302820. Epub 2015 May 18. Biomed Res Int. 2015. PMID: 26090397 Free PMC article.
References
-
- Owen TA, Aronow M, Shalhoub V, Barone LM, Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian LB, Stein GS. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol. 1990;143:420–30. - PubMed
-
- Rodan GA. Introduction to bone biology. Bone. 1992;13 (Suppl 1):S3–6. - PubMed
-
- Mornet E, Stura E, Lia-Baldini AS, Stigbrand T, Menez A, Le Du MH. Structural evidence for a functional role of human tissue nonspecific alkaline phosphatase in bone mineralization. J Biol Chem. 2001;276:31171–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

