Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming
- PMID: 12925677
- PMCID: PMC2194169
- DOI: 10.1084/jem.20030448
Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming
Abstract
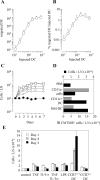
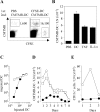
Antigen-pulsed dendritic cells (DCs) are used as natural adjuvants for vaccination, but the factors that influence the efficacy of this treatment are poorly understood. We investigated the parameters that affect the migration of subcutaneously injected mouse-mature DCs to the draining lymph node. We found that the efficiency of DC migration varied with the number of injected DCs and that CCR7+/+ DCs migrating to the draining lymph node, but not CCR7-/- DCs that failed to do so, efficiently induced a rapid increase in lymph node cellularity, which was observed before the onset of T cell proliferation. We also report that DC migration could be increased up to 10-fold by preinjection of inflammatory cytokines that increased the expression of the CCR7 ligand CCL21 in lymphatic endothelial cells. The magnitude and quality of CD4+ T cell response was proportional to the number of antigen-carrying DCs that reached the lymph node and could be boosted up to 40-fold by preinjection of tumor necrosis factor that conditioned the tissue for increased DC migration. These results indicate that DC number and tissue inflammation are critical parameters for DC-based vaccination.
Figures



References
-
- Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. - PubMed
-
- Sallusto, F., P. Schaerli, P. Loetscher, C. Schaniel, D. Lenig, C.R. Mackay, S. Qin, and A. Lanzavecchia. 1998. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur. J. Immunol. 28:2760–2769. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

