Messenger RNA reprogramming by spliceosome-mediated RNA trans-splicing
- PMID: 12925685
- PMCID: PMC171395
- DOI: 10.1172/JCI19462
Messenger RNA reprogramming by spliceosome-mediated RNA trans-splicing
Abstract
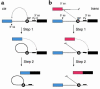
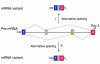
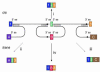
In the human genome, the majority of protein-encoding genes are interrupted by introns, which are removed from primary transcripts by a macromolecular enzyme known as the spliceosome. Spliceosomes can constitutively remove all the introns in a primary transcript to yield a fully spliced mRNA or alternatively splice primary transcripts leading to the production of many different mRNAs from one gene. This review examines how spliceosomes can recombine two primary transcripts in trans to reprogram messenger RNAs.
Figures



References
-
- Kole R, Sazani P. Antisense effects in the nucleus: modification of splicing. Curr. Opin. Mol. Ther. 2001;3:229–234. - PubMed
-
- Sullenger BA, Gilboa E. Emerging clinical applications of RNA. Nature. 2002;418:252–258. - PubMed
-
- Garcia-Blanco MA, Puttaraju M, Mansfield SG, Mitchell LG. Spliceosome-mediated RNA trans-splicing in gene therapy and genomics. Gene Ther. Reg. 2000;1:141–163. - PubMed
-
- Sullenger B, Cech TR. Ribozyme-mediated repair of defective mRNA by targeted trans-splicing. Nature. 1994;317:619–622. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

