Cathepsin V is involved in the degradation of invariant chain in human thymus and is overexpressed in myasthenia gravis
- PMID: 12925692
- PMCID: PMC171390
- DOI: 10.1172/JCI18028
Cathepsin V is involved in the degradation of invariant chain in human thymus and is overexpressed in myasthenia gravis
Abstract
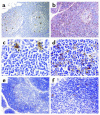
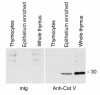
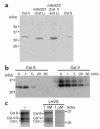
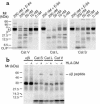
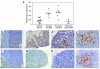
Stepwise degradation of the invariant chain (Ii) is required for the binding of antigenic peptides to MHC class II molecules. Cathepsin (Cat) L in the murine thymus and Cat S in peripheral APCs have both been implicated in the last step of Ii degradation that gives rise to the class II-associated invariant chain peptides (CLIP). Cat V has been recently described as highly homologous to Cat L and exclusively expressed in human thymus and testis, but with no mouse orthologue. We report that Cat V is the dominant cysteine protease in cortical human thymic epithelial cells, while Cat L and Cat S seem to be restricted to dendritic and macrophage-like cells. Active Cat V in thymic lysosomal preparations was demonstrated by active-site labeling. Recombinant Cat V was capable of converting Ii into CLIP efficiently, suggesting that Cat V is the protease that controls the generation of alphabeta-CLIP complexes in the human thymus, in analogy to Cat L in mouse. Comparison of Cat V expression between thymi from patients with myasthenia gravis and healthy controls revealed a significantly higher expression level in the pathological samples, suggesting a potential involvement of this protease in the immunopathogenesis of myasthenia gravis, an autoimmune disease almost invariably associated with thymic pathology.
Figures


References
-
- Riese RJ, Chapman HA. Cathepsins and compartmentalization in antigen presentation. Curr. Opin. Immunol. 2000;12:107–113. - PubMed
-
- Villadangos JA, Ploegh HL. Proteolysis in MHC class II antigen presentation: who’s in charge? Immunity. 2002;12:233–239. - PubMed
-
- Manoury B, et al. An asparaginyl endopeptidase processes a microbial antigen for class II MHC presentation [see comments] Nature. 1998;396:695–699. - PubMed
-
- Driessen C, Lennon-Dumenil AM, Ploegh HL. Individual cathepsins degrade immune complexes internalized by antigen- presenting cells via Fcgamma receptors. Eur. J. Immunol. 2001;31:1592–1601. - PubMed
-
- Cresswell P. Assembly, transport, and function of MHC class II molecules. Annu. Rev. Immunol. 1994;12:259–293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

