Collecting duct-specific gene inactivation of alphaENaC in the mouse kidney does not impair sodium and potassium balance
- PMID: 12925696
- PMCID: PMC171384
- DOI: 10.1172/JCI16956
Collecting duct-specific gene inactivation of alphaENaC in the mouse kidney does not impair sodium and potassium balance
Abstract
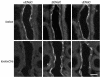
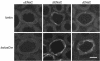
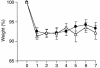
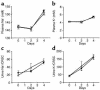
Aldosterone controls the final sodium reabsorption and potassium secretion in the kidney by regulating the activity of the epithelial sodium channel (ENaC) in the aldosterone-sensitive distal nephron (ASDN). ASDN consists of the last portion of the distal convoluted tubule (late DCT), the connecting tubule (CNT), and the collecting duct (CD) (i.e., the cortical CD [CCD] and the medullary CD [MCD]). It has been proposed that the control of sodium transport in the CCD is essential for achieving sodium and potassium balance. We have tested this hypothesis by inactivating the alpha subunit of ENaC in the CD but leaving ENaC expression in the late DCT and CNT intact. Under salt restriction or under aldosterone infusion, whole-cell voltage clamp of principal cells of CCD showed no detectable ENaC activity, whereas large amiloride-sensitive currents were observed in control littermates. The animals survive well and are able to maintain sodium and potassium balance, even when challenged by salt restriction, water deprivation, or potassium loading. We conclude that the expression of ENaC in the CD is not a prerequisite for achieving sodium and potassium balance in mice. This stresses the importance of more proximal nephron segments (late DCT/CNT) to achieve sodium and potassium balance.
Figures



References
-
- Verrey, F., Hummler, E., Schild, L., and Rossier, B.C. 2000. Control of Na+ transport by aldosterone. In The kidney: physiology and pathophysiology. Volume 1. Third edition. D.W. Seldin and G. Giebisch, eds. Lippincott, Williams, and Wilkins. Philadelphia, Pennsylvania, USA. 1441–1471.
-
- Canessa CM, et al. The amiloride-sensitive epithelial sodium channel is made of three homologous subunits. Nature. 1994;367:463–467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

