Beta-arrestin-2 regulates the development of allergic asthma
- PMID: 12925697
- PMCID: PMC171386
- DOI: 10.1172/JCI17265
Beta-arrestin-2 regulates the development of allergic asthma
Abstract
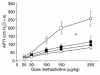
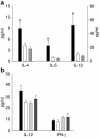
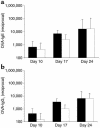
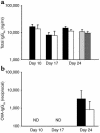
Asthma is a chronic inflammatory disorder of the airways that is coordinated by Th2 cells in both human asthmatics and animal models of allergic asthma. Migration of Th2 cells to the lung is key to their inflammatory function and is regulated in large part by chemokine receptors, members of the seven-membrane-spanning receptor family. It has been reported recently that T cells lacking beta-arrestin-2, a G protein-coupled receptor regulatory protein, demonstrate impaired migration in vitro. Here we show that allergen-sensitized mice having a targeted deletion of the beta-arrestin-2 gene do not accumulate T lymphocytes in their airways, nor do they demonstrate other physiological and inflammatory features characteristic of asthma. In contrast, the airway inflammatory response to LPS, an event not coordinated by Th2 cells, is fully functional in mice lacking beta-arrestin-2. beta-arrestin-2-deficient mice demonstrate OVA-specific IgE responses, but have defective macrophage-derived chemokine-mediated CD4+ T cell migration to the lung. This report provides the first evidence that beta-arrestin-2 is required for the manifestation of allergic asthma. Because beta-arrestin-2 regulates the development of allergic inflammation at a proximal step in the inflammatory cascade, novel therapies focused on this protein may prove useful in the treatment of asthma.
Figures

References
-
- Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu. Rev. Immunol. 1999;17:255–281. - PubMed
-
- National Institutes of Health. 1995. Data fact sheet: asthma statistics. Report number 55–798. National Heart Lung and Blood Institute. Washington, DC, USA. 1–4.
-
- Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu. Rev. Immunol. 2000;18:593–620. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

