Antibody blockade of the Cripto CFC domain suppresses tumor cell growth in vivo
- PMID: 12925698
- PMCID: PMC171388
- DOI: 10.1172/JCI17788
Antibody blockade of the Cripto CFC domain suppresses tumor cell growth in vivo
Abstract
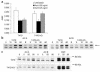
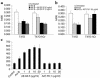
Cripto, a cell surface-associated protein belonging to the EGF-CFC family of growth factor-like molecules, is overexpressed in many human solid tumors, including 70-80% of breast and colon tumors, yet how it promotes cell transformation is unclear. During embryogenesis, Cripto complexes with Alk4 via its unique cysteine-rich CFC domain to facilitate signaling by the TGF-beta ligand Nodal. We report, for the first time to our knowledge, that Cripto can directly bind to another TGF-beta ligand, Activin B, and that Cripto overexpression blocks Activin B growth inhibition of breast cancer cells. This result suggests a novel mechanism for antagonizing Activin signaling that could promote tumorigenesis by deregulating growth homeostasis. We show that an anti-CFC domain antibody, A8.G3.5, both disrupts Cripto-Nodal signaling and reverses Cripto blockade of Activin B-induced growth suppression by blocking Cripto's association with either Alk4 or Activin B. In two xenograft models, testicular and colon cancer, A8.G3.5 inhibited tumor cell growth by up to 70%. Both Nodal and Activin B expression was found in the xenograft tumor, suggesting that either ligand could be promoting tumorigenesis. These data validate that functional blockade of Cripto inhibits tumor growth and highlight antibodies that block Cripto signaling mediated through its CFC domain as an important class of antibodies for further therapeutic development.
Figures





Comment in
-
Decrypting the role of Cripto in tumorigenesis.J Clin Invest. 2003 Aug;112(4):500-2. doi: 10.1172/JCI19546. J Clin Invest. 2003. PMID: 12925690 Free PMC article. Review.
References
-
- de Bono JS, Rowinsky EK. The ErbB receptor family: a therapeutic target for cancer. Trends Mol. Med. 2002;8(Suppl. 4):S19–S26. - PubMed
-
- George D. Platelet-derived growth factor receptors: a therapeutic target in solid tumors. Semin. Oncol. 2001;28:27–33. - PubMed
-
- Adamson ED, Minchiotti G, Salomon DS. Cripto: a tumor growth factor and more. J. Cell. Physiol. 2002;190:267–278. - PubMed
-
- Ciardiello F, et al. Inhibition of CRIPTO expression and tumorigenicity in human colon cancer cells by antisense RNA and oligodeoxynucleotides. Oncogene. 1994;9:291–298. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

