Muscle-specific PPARgamma-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones
- PMID: 12925701
- PMCID: PMC171387
- DOI: 10.1172/JCI17305
Muscle-specific PPARgamma-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones
Abstract
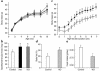
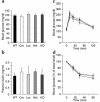
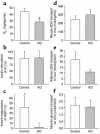
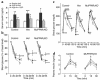
Activation of peroxisome proliferator-activated receptor gamma (PPARgamma) by thiazolidinediones (TZDs) improves insulin resistance by increasing insulin-stimulated glucose disposal in skeletal muscle. It remains debatable whether the effect of TZDs on muscle is direct or indirect via adipose tissue. We therefore generated mice with muscle-specific PPARgamma knockout (MuPPARgammaKO) using Cre/loxP recombination. Interestingly, MuPPARgammaKO mice developed excess adiposity despite reduced dietary intake. Although insulin-stimulated glucose uptake in muscle was not impaired, MuPPARgammaKO mice had whole-body insulin resistance with a 36% reduction (P < 0.05) in the glucose infusion rate required to maintain euglycemia during hyperinsulinemic clamp, primarily due to dramatic impairment in hepatic insulin action. When placed on a high-fat diet, MuPPARgammaKO mice developed hyperinsulinemia and impaired glucose homeostasis identical to controls. Simultaneous treatment with TZD ameliorated these high fat-induced defects in MuPPARgammaKO mice to a degree identical to controls. There was also altered expression of several lipid metabolism genes in the muscle of MuPPARgammaKO mice. Thus, muscle PPARgamma is not required for the antidiabetic effects of TZDs, but has a hitherto unsuspected role for maintenance of normal adiposity, whole-body insulin sensitivity, and hepatic insulin action. The tissue crosstalk mediating these effects is perhaps due to altered lipid metabolism in muscle.
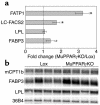
Figures

References
-
- Martin BC, et al. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet. 1992;340:925–929. - PubMed
-
- Lillioja S, et al. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus: prospective studies of Pima Indians. N. Engl. J. Med. 1993;329:1988–1992. - PubMed
-
- DeFronzo RA, et al. The effect of insulin on the disposal of intravenous glucose: results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. - PubMed
-
- Veroni MC, Proietto J, Larkins RG. Evolution of insulin resistance in New Zealand obese mice. Diabetes. 1991;40:1480–1487. - PubMed
-
- Petersen KF, et al. Mechanism of troglitazone action in type 2 diabetes. Diabetes. 2000;49:827–831. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

