Ankyrin binding mediates L1CAM interactions with static components of the cytoskeleton and inhibits retrograde movement of L1CAM on the cell surface
- PMID: 12925712
- PMCID: PMC2173803
- DOI: 10.1083/jcb.200211011
Ankyrin binding mediates L1CAM interactions with static components of the cytoskeleton and inhibits retrograde movement of L1CAM on the cell surface
Abstract
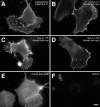
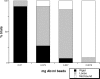
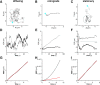
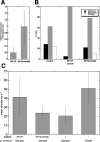
The function of adhesion receptors in both cell adhesion and migration depends critically on interactions with the cytoskeleton. During cell adhesion, cytoskeletal interactions stabilize receptors to strengthen adhesive contacts. In contrast, during cell migration, adhesion proteins are believed to interact with dynamic components of the cytoskeleton, permitting the transmission of traction forces through the receptor to the extracellular environment. The L1 cell adhesion molecule (L1CAM), a member of the Ig superfamily, plays a crucial role in both the migration of neuronal growth cones and the static adhesion between neighboring axons. To understand the basis of L1CAM function in adhesion and migration, we quantified directly the diffusion characteristics of L1CAM on the upper surface of ND-7 neuroblastoma hybrid cells as an indication of receptor-cytoskeleton interactions. We find that cell surface L1CAM engages in diffusion, retrograde movement, and stationary behavior, consistent with interactions between L1CAM and two populations of cytoskeleton proteins. We provide evidence that the cytoskeletal adaptor protein ankyrin mediates stationary behavior while inhibiting the actin-dependent retrograde movement of L1CAM. Moreover, inhibitors of L1CAM-ankyrin interactions promote L1CAM-mediated axon growth. Together, these results suggest that ankyrin binding plays a crucial role in the anti-coordinate regulation of L1CAM-mediated adhesion and migration.
Figures


References
-
- Bretscher, A., K. Edwards, and R.G. Fehon. 2002. ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 3:586–599. - PubMed
-
- Choquet, D., D.P. Felsenfeld, and M.P. Sheetz. 1997. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 88:39–48. - PubMed
-
- Cohen, N.R., J.S. Taylor, L.B. Scott, R.W. Guillery, P. Soriano, and A.J. Furley. 1998. Errors in corticospinal axon guidance in mice lacking the neural cell adhesion molecule L1. Curr. Biol. 8:26–33. - PubMed
-
- Dahme, M., U. Bartsch, R. Martini, B. Anliker, M. Schachner, and N. Mantei. 1997. Disruption of the mouse L1 gene leads to malformations of the nervous system. Nat. Genet. 17:346–349. - PubMed
-
- Davis, J.Q., and V. Bennett. 1994. Ankyrin binding activity shared by the neurofascin/L1/NrCAM family of nervous system cell adhesion molecules. J. Biol. Chem. 269:27163–27166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

