Evidence for a primary islet autoantigen (preproinsulin 1) for insulitis and diabetes in the nonobese diabetic mouse
- PMID: 12925730
- PMCID: PMC193569
- DOI: 10.1073/pnas.1834450100
Evidence for a primary islet autoantigen (preproinsulin 1) for insulitis and diabetes in the nonobese diabetic mouse
Abstract
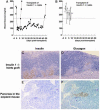
It has been reported that an insulin 2 gene knockout, when bred onto nonobese diabetic (NOD) mice, accelerates diabetes. We produced insulin 1 gene knockout congenic NOD mice. In contrast to insulin 2, diabetes and insulitis were markedly reduced in insulin 1 knockout mice, with decreased and delayed diabetes in heterozygous females and no insulitis and diabetes in most homozygous female mice. Lack of insulitis was found for insulin 1 female homozygous knockout mice at 8, 12, and 37 weeks of age. Despite a lack of insulitis, insulin 1 homozygous knockout mice spontaneously expressed insulin autoantibodies. Administration of insulin peptide B:9-23 of both insulin 1 and 2 to NOD mice induced insulin autoantibodies. Insulin 1 is not the only lymphocytic target of NOD mice. Insulin 1 homozygous knockout islets, when transplanted into recently diabetic wild-type NOD mice, became infiltrated with lymphocytes and only transiently reversed diabetes. These observations indicate that loss of either insulin gene can influence progression to diabetes of NOD mice and suggest that the preproinsulin 1 gene is crucial for the spontaneous development of NOD insulitis and diabetes.
Figures
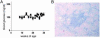


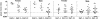
References
-
- Rose, N. R. & Bona, C. (1993) Immunol. Today 14, 426-430. - PubMed
-
- Wucherpfennig, K. W. & Eisenbarth, G. S. (2001) Nat. Immunol. 2, 1-3. - PubMed
-
- Hattori, M., Buse, J. B., Jackson, R. A., Glimcher, L., Dorf, M. E., Minami, M., Makino, S., Moriwaki, K., Korff, M., Kuzuya, H., et al. (1986) Science 231, 733-735. - PubMed
-
- Todd, J. A., Acha-Orbea, H., Bell, J. I., Chao, N., Fronek, Z., Jacob, C. O., McDermott, M., Sinha, A. A., Timmerman, L., Steinman, L., et al. (1988) Science 240, 1003-1009. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK057516/DK/NIDDK NIH HHS/United States
- R01 DK062718/DK/NIDDK NIH HHS/United States
- AI95380/AI/NIAID NIH HHS/United States
- DK32493/DK/NIDDK NIH HHS/United States
- DK50970/DK/NIDDK NIH HHS/United States
- DK32082/DK/NIDDK NIH HHS/United States
- R01 DK055969/DK/NIDDK NIH HHS/United States
- DK55969/DK/NIDDK NIH HHS/United States
- R37 DK032493/DK/NIDDK NIH HHS/United States
- P30 DK57516/DK/NIDDK NIH HHS/United States
- DK62718/DK/NIDDK NIH HHS/United States
- AI46374/AI/NIAID NIH HHS/United States
- U19 AI050864/AI/NIAID NIH HHS/United States
- AI39213/AI/NIAID NIH HHS/United States
- U19 AI046374/AI/NIAID NIH HHS/United States
- AI50864/AI/NIAID NIH HHS/United States
- R01 DK032493/DK/NIDDK NIH HHS/United States
- R01 AI039213/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

