Alzheimer's disease-affected brain: presence of oligomeric A beta ligands (ADDLs) suggests a molecular basis for reversible memory loss
- PMID: 12925731
- PMCID: PMC193576
- DOI: 10.1073/pnas.1834302100
Alzheimer's disease-affected brain: presence of oligomeric A beta ligands (ADDLs) suggests a molecular basis for reversible memory loss
Abstract
A molecular basis for memory failure in Alzheimer's disease (AD) has been recently hypothesized, in which a significant role is attributed to small, soluble oligomers of amyloid beta-peptide (A beta). A beta oligomeric ligands (also known as ADDLs) are known to be potent inhibitors of hippocampal long-term potentiation, which is a paradigm for synaptic plasticity, and have been linked to synapse loss and reversible memory failure in transgenic mouse AD models. If such oligomers were to build up in human brain, their neurological impact could provide the missing link that accounts for the poor correlation between AD dementia and amyloid plaques. This article, using antibodies raised against synthetic A beta oligomers, verifies the predicted accumulation of soluble oligomers in AD frontal cortex. Oligomers in AD reach levels up to 70-fold over control brains. Brain-derived and synthetic oligomers show structural equivalence with respect to mass, isoelectric point, and recognition by conformation-sensitive antibodies. Both oligomers, moreover, exhibit the same striking patterns of attachment to cultured hippocampal neurons, binding on dendrite surfaces in small clusters with ligand-like specificity. Binding assays using solubilized membranes show oligomers to be high-affinity ligands for a small number of nonabundant proteins. Current results confirm the prediction that soluble oligomeric A beta ligands are intrinsic to AD pathology, and validate their use in new approaches to therapeutic AD drugs and vaccines.
Figures
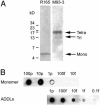

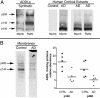


References
-
- Hardy, J. & Selkoe, D. J. (2002) Science 297, 353-356. - PubMed
-
- Kirkitadze, M. D., Bitan, G. & Teplow, D. B. (2002) J. Neurosci. Res. 69, 567-577. - PubMed
-
- Klein, W. L. (2000) in Molecular Mechanisms of Neurodegenerative Diseases, ed. Chesselet, M.-F. (Humana, Totowa, NJ), pp. 1-49.
-
- Kang, J., Lemaire, H. G., Unterbeck, A., Salbaum, J. M., Masters, C. L., Grzeschik, K. H., Multhaup, G., Beyreuther, K. & Muller-Hill, B. (1987) Nature 325, 733-736. - PubMed
-
- Goate, A., Chartier-Harlin, M. C., Mullan, M., Brown, J., Crawford, F., Fidani, L., Giuffra, L., Haynes, A., Irving, N. & James, L. (1991) Nature 349, 704-706. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

