Activity and distribution of paxillin, focal adhesion kinase, and cadherin indicate cooperative roles during zebrafish morphogenesis
- PMID: 12925747
- PMCID: PMC181551
- DOI: 10.1091/mbc.e02-08-0537
Activity and distribution of paxillin, focal adhesion kinase, and cadherin indicate cooperative roles during zebrafish morphogenesis
Abstract
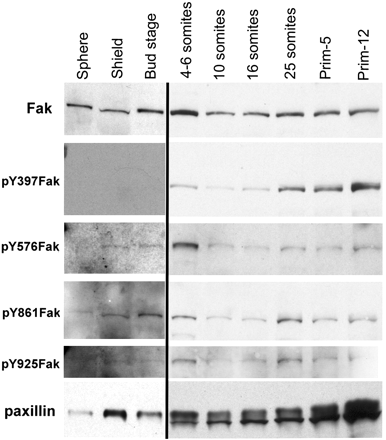
We investigated the focal adhesion proteins paxillin and Fak, and the cell-cell adhesion protein cadherin in developing zebrafish (Danio rerio) embryos. Cadherins are expressed in presomitic mesoderm where they delineate cells. The initiation of somite formation coincides with an increase in the phosphorylation of Fak, and the accumulation of Fak, phosphorylated Fak, paxillin, and fibronectin at nascent somite boundaries. In the notochord, cadherins are expressed on cells during intercalation, and phosphorylated Fak accumulates in circumferential rings where the notochord cells contact laminin in the perichordal sheath. Subsequently, changes in the orientations of collagen fibers in the sheath suggest that Fak-mediated adhesion allows longitudinal expansion of the notochord, but not lateral expansion, resulting in notochord elongation. Novel observations showed that focal adhesion kinase and paxillin concentrate at sites of cell-cell adhesion in the epithelial enveloping layer and may associate with actin cytoskeleton at epithelial junctions containing cadherins. Fak is phosphorylated at these epithelial junctions but is not phosphorylated on Tyr397, implicating a noncanonical mechanism of regulation. These data suggest that Fak and paxillin may function in the integration of cadherin-based and integrin-based cell adhesion during the morphogenesis of the early zebrafish embryo.
Figures








Similar articles
-
Roles for zebrafish focal adhesion kinase in notochord and somite morphogenesis.Dev Biol. 2001 Dec 15;240(2):474-87. doi: 10.1006/dbio.2001.0467. Dev Biol. 2001. PMID: 11784077
-
Paxillin null embryonic stem cells are impaired in cell spreading and tyrosine phosphorylation of focal adhesion kinase.Oncogene. 2002 Jan 3;21(1):96-107. doi: 10.1038/sj.onc.1205013. Oncogene. 2002. PMID: 11791180
-
Adenovirus-mediated overexpression of C-terminal Src kinase (Csk) in type I astrocytes interferes with cell spreading and attachment to fibronectin. Correlation with tyrosine phosphorylations of paxillin and FAK.J Biol Chem. 1999 Jan 22;274(4):2291-7. doi: 10.1074/jbc.274.4.2291. J Biol Chem. 1999. PMID: 9890993
-
FAK and paxillin: regulators of N-cadherin adhesion and inhibitors of cell migration?J Cell Biol. 2004 Jul 19;166(2):157-9. doi: 10.1083/jcb.200406151. J Cell Biol. 2004. PMID: 15263014 Free PMC article. Review.
-
Transmembrane control of cadherin-mediated cell-cell adhesion.Semin Cell Biol. 1993 Jun;4(3):175-81. doi: 10.1006/scel.1993.1021. Semin Cell Biol. 1993. PMID: 8347834 Review.
Cited by
-
Activity-based labeling of matrix metalloproteinases in living vertebrate embryos.PLoS One. 2012;7(8):e43434. doi: 10.1371/journal.pone.0043434. Epub 2012 Aug 28. PLoS One. 2012. PMID: 22952682 Free PMC article.
-
NAD+ biosynthesis ameliorates a zebrafish model of muscular dystrophy.PLoS Biol. 2012;10(10):e1001409. doi: 10.1371/journal.pbio.1001409. Epub 2012 Oct 23. PLoS Biol. 2012. PMID: 23109907 Free PMC article.
-
Time-lapse analysis and mathematical characterization elucidate novel mechanisms underlying muscle morphogenesis.PLoS Genet. 2008 Oct 3;4(10):e1000219. doi: 10.1371/journal.pgen.1000219. PLoS Genet. 2008. PMID: 18833302 Free PMC article.
-
FAK is required for tension-dependent organization of collective cell movements in Xenopus mesendoderm.Dev Biol. 2014 Oct 15;394(2):340-56. doi: 10.1016/j.ydbio.2014.07.023. Epub 2014 Aug 13. Dev Biol. 2014. PMID: 25127991 Free PMC article.
-
Csrp1 regulates dynamic cell movements of the mesendoderm and cardiac mesoderm through interactions with Dishevelled and Diversin.Proc Natl Acad Sci U S A. 2007 Jul 3;104(27):11274-9. doi: 10.1073/pnas.0702000104. Epub 2007 Jun 25. Proc Natl Acad Sci U S A. 2007. PMID: 17592114 Free PMC article.
References
-
- Adams, D.S., Keller, R., and Ma, K. (1990). The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development 110, 115–130. - PubMed
-
- Avizienyte, E., Wyke, A.W., Jones, R.J., McLean, G.W., Westhoff, M.A., Brunton, V.G., and Frame, M.C. (2002). Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signaling. Nat. Cell Biol. 4, 632–638. - PubMed
-
- Babb, S.G., Barnett, J., Doedens, A.L., Cobb, N., Liu, Q., Sorkin, B.C., Yelick, P.C., Raymond, P.A., and Marrs, J.A. (2001). Zebrafish E-cadherin: expression during early embryogenesis and regulation during brain development. Dev. Dyn. 22, 231–237. - PubMed
-
- Barrantes, I.B., Elia, A.J., Wunsch, K., De Angelis, M.H., Mak, T.W., Rossant, J., Conlon, R.A., Gossler, A., and de la Pompa, J.L. (1999). Interaction between notch signaling and lunatic fringe during somite boundary formation in the mouse. Curr. Biol. 9, 470–480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

