The tumor suppressor cybL, a component of the respiratory chain, mediates apoptosis induction
- PMID: 12925748
- PMCID: PMC181552
- DOI: 10.1091/mbc.e02-10-0631
The tumor suppressor cybL, a component of the respiratory chain, mediates apoptosis induction
Abstract
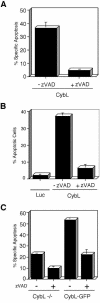
A genetic screen was established to clone apoptosis-inducing genes in a high-throughput format. It led to the isolation of several proapoptotic genes whose proteins are localized to mitochondria. One of the isolated genes is cytochrome bL (cybL also known as SDHC, CII-3, or QPs-1), a component of the respiratory chain complex II. It was further investigated because both cybL and another component of complex II, cybS, have recently been identified as tumor suppressor proteins, some of which act by controlling apoptosis. Our studies reveal that cell death induction by cybL expression is concomitant with a transient inhibition of complex II and the generation of reactive oxygen species. Importantly, cells that are constitutively deficient in cybL are resistant to a variety of proapoptotic cytostatic drugs and to the effects of the Fas receptor. Our results therefore identify complex II as a sensor for apoptosis induction and could explain the unexpected observation that complex II is inactivated in tumors.
Figures


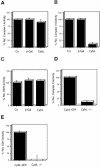
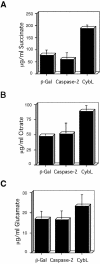
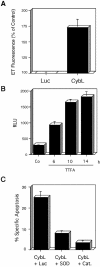

References
-
- Baysal, B.E., et al. (2000). Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 287, 848–851. - PubMed
-
- Beere, H.M., Wolf, B.B., Cain, K., Mosser, D.D., Mahboubi, A., Kuwana, T., Tailor, P., Morimoto, R.I., Cohen, G.M., and Green, D.R. (2000). Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2, 469–475. - PubMed
-
- Bejaoui, K., Wu, C., Scheffler, M.D., Haan, G., Ashby, P., Wu, L., de Jong, P., and Brown, R.H. (2001). SPTLC1 is mutated in hereditary sensory neuropathy, type 1. Nat. Genet. 27, 261–262. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

