Suppression of coatomer mutants by a new protein family with COPI and COPII binding motifs in Saccharomyces cerevisiae
- PMID: 12925749
- PMCID: PMC181553
- DOI: 10.1091/mbc.e02-11-0736
Suppression of coatomer mutants by a new protein family with COPI and COPII binding motifs in Saccharomyces cerevisiae
Abstract
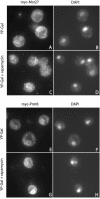
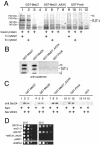
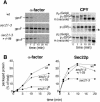
Protein trafficking is achieved by a bidirectional vesicle flow between the various compartments of the eukaryotic cell. COPII coated vesicles mediate anterograde protein transport from the endoplasmic reticulum to the Golgi apparatus, whereas retrograde Golgi-to-endoplasmic reticulum vesicles use the COPI coat. Inactivation of COPI vesicle formation in conditional sec21 (gamma-COP) mutants rapidly blocks transport of certain proteins along the early secretory pathway. We have identified the integral membrane protein Mst27p as a strong suppressor of sec21-3 and ret1-1 mutants. A C-terminal KKXX motif of Mst27p that allows direct binding to the COPI complex is crucial for its suppression ability. Mst27p and its homolog Yar033w (Mst28p) are part of the same complex. Both proteins contain cytoplasmic exposed C termini that have the ability to interact directly with COPI and COPII coat complexes. Site-specific mutations of the COPI binding domain abolished suppression of the sec21 mutants. Our results indicate that overexpression of MST27 provides an increased number of coat binding sites on membranes of the early secretory pathway and thereby promotes vesicle formation. As a consequence, the amount of cargo that can bind COPI might be important for the regulation of the vesicle flow in the early secretory pathway.
Figures






Similar articles
-
COPI-independent anterograde transport: cargo-selective ER to Golgi protein transport in yeast COPI mutants.J Cell Biol. 1997 Feb 24;136(4):789-802. doi: 10.1083/jcb.136.4.789. J Cell Biol. 1997. PMID: 9049245 Free PMC article.
-
Requirement for neo1p in retrograde transport from the Golgi complex to the endoplasmic reticulum.Mol Biol Cell. 2003 Dec;14(12):4971-83. doi: 10.1091/mbc.e03-07-0463. Epub 2003 Sep 5. Mol Biol Cell. 2003. PMID: 12960419 Free PMC article.
-
Auxilin facilitates membrane traffic in the early secretory pathway.Mol Biol Cell. 2016 Jan 1;27(1):127-36. doi: 10.1091/mbc.E15-09-0631. Epub 2015 Nov 4. Mol Biol Cell. 2016. PMID: 26538028 Free PMC article.
-
Formation of COPI-coated vesicles at a glance.J Cell Sci. 2018 Mar 13;131(5):jcs209890. doi: 10.1242/jcs.209890. J Cell Sci. 2018. PMID: 29535154 Review.
-
COPI budding within the Golgi stack.Cold Spring Harb Perspect Biol. 2011 Nov 1;3(11):a005231. doi: 10.1101/cshperspect.a005231. Cold Spring Harb Perspect Biol. 2011. PMID: 21844168 Free PMC article. Review.
Cited by
-
Adaptive laboratory evolution of β-caryophyllene producing Saccharomyces cerevisiae.Microb Cell Fact. 2021 May 27;20(1):106. doi: 10.1186/s12934-021-01598-z. Microb Cell Fact. 2021. PMID: 34044821 Free PMC article.
-
Statistical analysis reveals co-expression patterns of many pairs of genes in yeast are jointly regulated by interacting loci.PLoS Genet. 2013 Mar;9(3):e1003414. doi: 10.1371/journal.pgen.1003414. Epub 2013 Mar 28. PLoS Genet. 2013. PMID: 23555313 Free PMC article.
-
Novel cargo-binding site in the beta and delta subunits of coatomer.J Cell Biol. 2007 Oct 22;179(2):209-17. doi: 10.1083/jcb.200704142. J Cell Biol. 2007. PMID: 17954604 Free PMC article.
-
Protein sorting at the ER-Golgi interface.J Cell Biol. 2016 Dec 19;215(6):769-778. doi: 10.1083/jcb.201610031. Epub 2016 Nov 30. J Cell Biol. 2016. PMID: 27903609 Free PMC article. Review.
-
Organization of physical interactomes as uncovered by network schemas.PLoS Comput Biol. 2008 Oct;4(10):e1000203. doi: 10.1371/journal.pcbi.1000203. Epub 2008 Oct 24. PLoS Comput Biol. 2008. PMID: 18949022 Free PMC article.
References
-
- Aridor, M., and Traub, L.M. (2002). Cargo selection in vesicular transport: the making and breaking of a coat. Traffic 3, 537–546. - PubMed
-
- Arnott, D., O'Connell, K.L., King, K.L., and Stults, J.T. (1998). An integrated approach to proteome analysis: identification of proteins associated with cardiac hypertrophy. Anal. Biochem. 258, 1–18. - PubMed
-
- Baker, D., Hicke, L., Rexach, M., Schleyer, M., and Schekman, R. (1988). Reconstitution of SEC gene product-dependent intercompartmental protein transport. Cell 54, 335–344. - PubMed
-
- Barlowe, C., Orci, L., Yeung, T., Hosobuchi, M., Hamamoto, S., Salama, N., Rexach, M.F., Ravazzola, M., Amherdt, M., and Schekman, R. (1994). COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77, 895–907. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

