Subcellular localization of LGN during mitosis: evidence for its cortical localization in mitotic cell culture systems and its requirement for normal cell cycle progression
- PMID: 12925752
- PMCID: PMC181556
- DOI: 10.1091/mbc.e03-04-0212
Subcellular localization of LGN during mitosis: evidence for its cortical localization in mitotic cell culture systems and its requirement for normal cell cycle progression
Abstract
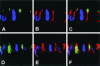
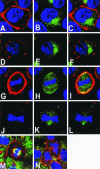
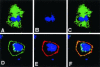
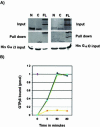
Mammalian LGN/AGS3 proteins and their Drosophila Pins orthologue are cytoplasmic regulators of G-protein signaling. In Drosophila, Pins localizes to the lateral cortex of polarized epithelial cells and to the apical cortex of neuroblasts where it plays important roles in their asymmetric division. Using overexpression studies in different cell line systems, we demonstrate here that, like Drosophila Pins, LGN can exhibit enriched localization at the cell cortex, depending on the cell cycle and the culture system used. We find that in WISH, PC12, and NRK but not COS cells, LGN is largely directed to the cell cortex during mitosis. Overexpression of truncated protein domains further identified the Galpha-binding C-terminal portion of LGN as a sufficient domain for cortical localization in cell culture. In mitotic COS cells that normally do not exhibit cortical LGN localization, LGN is redirected to the cell cortex upon overexpression of Galpha subunits of heterotrimeric G-proteins. The results also show that the cortical localization of LGN is dependent on microfilaments and that interfering with LGN function in cultured cell lines causes early disruption to cell cycle progression.
Figures
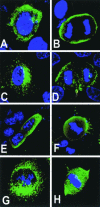
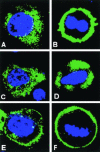
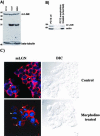
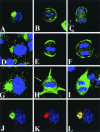
References
-
- Bernard, M.L., Peterson, Y.K., Chung, P., Jourdan, J., and Lanier, S.M. (2001). Selective interaction of AGS3 with G-proteins and the influence of AGS3 on the activation state of G-proteins. J. Biol. Chem. 276, 1585–1593. - PubMed
-
- Blatch, G.L., and Lassle, M. (1999). The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. Bioessays 21, 932–939. - PubMed
-
- Blumer, J.B., Chandler, L.J., and Lanier, S.M. (2002). Expression analysis and subcellular distribution of the two G-protein regulators AGS3 and LGN indicate distinct functionality. Localization of LGN to the midbody during cytokinesis. J. Biol. Chem. 277, 15897–15903. - PubMed
-
- Chen, C., Zheng, B., Han, J., and Lin, S.C. (1997). Characterization of novel RGS proteins that bind to Gα proteins and inhibits pheromone signalling in yeast. J. Biol. Chem. 272, 8679–8685. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

